Prevalence and Genotypic Distribution of Bovine Leukemia Virus across Asian Regions: Insights into Economic Significance and Clinical Staging
© 2025 Bio Communications
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Abstract
This study critically reviews the literature on the prevalence and genotypic distribution of Bovine Leukemia Virus (BLV) in Asia, specifically targeting enzootic bovine leukosis, a chronic disease affecting cattle’s life and milk production along with reduced fat yield, that causing economic losses due to increased susceptibility to pathogens. European countries are eradicating BLV but Asia has a high prevalence, particularly in Asian cattle. Enzootic bovine leukosis (EBL) has distinct clinical stages: asymptomatic, persistent lymphocytosis, and lymphosarcoma. It typically occurs in adult animals, with 60% being asymptomatic during the aleukemic stage, 30% developing persistent lymphocytosis, and 5% developing lymphoma. Databases like PubMed and Google Scholar were used to search molecular and prevalence studies from 2000-2024, focusing on Asian countries. Prevalence of bovine leukosis (BL) in Asia varies significantly, with high rates in central and eastern regions. BLV is a global disease, has increased in Asia with highest prevalence in central Asia about 40.0–84.0% (Kazakhstan), 35% in Eastern Asia (Japan), and 58.3% in Southeast Asia (Thailand). BLV genotypes G1, G4, G6, G7, G12, G3, G4, G6, G7, and G10 are prevalent in various regions of Asia, with G1 being most prevalent globally. These genotypes might be spread due to animal trading, and animal domestication, affecting various countries. This review provides the current status of enzootic bovine leukosis in Asia, emphasising the need for a closed trading system to control the disease.
Keywords:
Bovine Leukemia Virus (BLV), Genotype, Prevalence, Cattle, Asia
1. Introduction
Bovine leukemia virus is an oncogenic RNA virus belonging to the Deltaretrovirus genus of the Retroviridae family. It causes Enzootic Bovine Leukosis (EBL) and targets B cells, leading to B lymphocytotropic (Mousavi et al., 2014; Olaya-Galán et al., 2022). It is also known by other names like Bovine lymphomastitis, bovine lymphosarcoma or malignant lymphoma of cattle. It is included among the five important diseases of cattle. The virus was isolated for the first time in Germany. BLV can be transmitted vertically and horizontally through blood-contaminated procedures like shared needles, reproductive sleeves, and natural breeding (Kiugu, 2018). Vertical transmission includes intrauterine infection, particularly in dams with high viral loads (Sajiki et al., 2017) and through colostrum and milk (Quadros et al., 2024) . Horizontal transmission involves the transfer of infected cells via direct contact, insect bites, and contaminated iatrogenic procedures (Joris et al., 2021).
Leukosis was first reported by Leisering in 1871 when he found yellowish nodules and tumors in the body (Leisering, 1871). Bovine Leukosis (BL) is characterized by uncontrolled lymphocyte proliferation, immunosuppression, and tumor development in a variety of organs. It induces a lifetime infection by integrating its genetic material into host cells (Juliarena et al., 2017). One of the most crucial concerns about BLV infection is that it remains asymptomatic in multiple animal species. BLV infects all mammalian species, but cattle are more inherently infected due to the presence of AP3D1 receptors (Olaya-Galán et al., 2022). It is transmitted horizontally by blood (infected B lymphocytes), milk (especially colostrum), and intrauterine infection (Shrestha et al., 2024). Buffaloes were naturally infected and susceptible to infection when challenged with BLV. Countries pursuing the eradication program should screen buffaloes as they could be a permanent threat to free status if remain infected (Feliziani et al., 2017; Molnar et al., 2000; Romero et al., 1981). The majority of sheep that have been artificially infected died from leukemia (Florins et al., 2008).
BLV is a persistent infection, with 70% of infected animals remain asymptomatic, 30% developing lymphocytosis, and 10% developing leukemia/lymphoma (Barez et al., 2015). Only about one-third of the infected cattle experience persistent lymphocytosis and in 5% cases, it may evolve to lymphosarcoma (Trono et al., 2001). BL has a significant economic impact due to reduced milk production, diminished fertility, increased susceptibility to other diseases, and premature culling of infected animals (Nakada et al., 2022). EBLV leads to restrictions on international trade, which is a major concern for livestock due to its economic and animal welfare implications (WOAH). In Asia, there is a gap in research on bovine leukemia virus, and the notable lack of comprehensive studies on its prevalence and genetic diversity. Additionally clinical staging and economic loss due to enzootic bovine leukosis. The review summarizes recent research on BLV prevalence and molecular characterization in Asia, highlighting its significant economic impact and being crucial for cattle health and livestock.
2. Economical impact of BLV
Enzootic bovine leukosis is a chronic disease that has detrimental effects on cattle’s life and milk production (Nekouei et al., 2016). Economic losses due to enzootic bovine leukosis can decrease milk production, fertility, life span, and condemnation at slaughter (Khudhair et al., 2016a). Higher BLV prevalence has been associated with reduced herd milk production and a shortened cow’s lifespan (Erskine et al., 2012; Nekouei et al., 2016). BLV milk positivity was associated with loss of milk production at the individual cow level (305-d mature-equivalent yields), especially among the older cows (Norby et al., 2016). The association of subclinical mastitis with BLV infection was also investigated. Estimated economic loss per animal was $418.59 and the annual loss was $6,097,225 due to mastitis caused by BLV. These animals become more susceptible to pathogens due to immunosuppression. So, BLV infection causes a significant economic loss in the dairy industry (Nakada et al., 2023). On average, there was a loss per animal of about $59 in a BLV-positive herd and a 218kg milk reduction. About a 2.7% decline in milk production occurs in test-negative cows. These findings were quite comparable to earlier studies as well (Ott et al., 2003). Fat yield also seemed to be highly affected due to BLV, especially with progressing age and the condition of persistent lymphocytosis (PL). Over a period of 6 years, the annual economic loss was calculated to be $90.72 and $325.08 for animals having PL for 2 and 3 years, respectively (Da et al., 1993). In Michigan dairy herds, higher BLV prevalence has been associated with reduced herd milk production and a shortened cow lifespan (Erskine et al., 2012).
In Japan, about 21.6% of carcass weight reduction was occurring due to BLV infection. A US$1,391,649 significant economic loss resulting from carcass weight loss brought on by BLV infection was investigated (Nakada et al., 2022). Malignant lymphoma was causing 26.87% of dairy and 13.35% of beef carcass condemnation in the US about 274,029 of 7,138,997 cull dairy cows were condemned (White & Moore, 2009). The rate of condemned carcasses due to malignant lymphoma was higher during winter in California and US states (Amirpour Haredasht et al., 2018). BLV-positive animal were 23% more likely to be culled than their BLV-negative herd mates in 112 Michigan dairy herds, indicating its economic impact on US dairy (Bartlett et al., 2013). However, a study on a large dairy farm demonstrated successful BLV reduction through selective culling based on diagnostic testing, reducing BLV ELISA-positive cows to 0.85% after three years (Taxis et al., 2023).The association of subclinical mastitis with BLV infection was also investigated. EBLV leads to restrictions on international trade, which is a major concern for livestock due to its economic and animal welfare implications (WOAH).
3. Prevalence of BLV in Asian Countries
BLV has become prevalent all over the world, at various levels of prevalence. Some studies showed that Japan was the first Asian country where BLV was reported, but complete evidence is not present. The first case of BLV in Japan was reported in 1927 in Iwate Prefecture, and then gradually, cases increased (K. Nishikaku et al., 2022). Since then, Asian nations have been measuring the BLV prevalence. In Asian regions, Prevalence of BLV reported over the previous 24 years is shown in Table 1. In Western Asia, about 25.7% prevalence was calculated in dairy cattle of the UAE (Abu Dhabi) using ELISA (Hassan et al., 2020). In Turkey, BLV prevalence was calculated to be about 1.6% in 2003 based on the Immunodiffusion test and 21% in 2005 based on AGID (Burgu et al., 2005; Meas et al., 2003). In Kazakhstan (Central Asia), BLV seems to be at a high level of prevalence of about 40-84% overall based on PCR test results (Mamanova et al., 2020). In Eastern Asia, Japan has an overall 35% prevalence in both dairy and beef cattle measured in 2013 (Murakami et al., 2013). In Korea and China, BLV is reported at about 10.2% and 10% overall based on ELISA (Kim et al., 2017; Ma et al., 2021). In Taiwan again, a high prevalence of about 99% in dairy cattle at the herd level and 81.8% at the individual level was calculated in 2019 (Hsieh et al., 2019). In Mongolia, a 3.9% overall prevalence was reported based on PCR results of blood from dairy cattle in Tuv, Arkhangai, and Ulaanbaatar (N. Ochirkhuu et al., 2016). In southern Asia in 2017, India had about 9.09% overall prevalence in dairy and breeding cattle (Katoch et al., 2017). In Iran, the prevalence calculated in Isfahan and Bukhtiary provinces was about 22.1%–25.0% overall in cattle, sheep, and camel (with 0% in camel) (Nekoei et al., 2015). In Pakistan, 20% BLV prevalence is calculated in cattle from the Northwest region based on Indirect ELISA (Khan et al., 2020). The next region is Southeast Asia, in which 9 provinces of Thailand (2016) and 5 provinces of Cambodia (2000) measured a prevalence of 58.3% and 5.3%, respectively (Lee et al., 2016; Meas et al., 2000). The Philippines reported that 23.1% of cattle were affected by BLV in 2015, while Myanmar reported 37.4% of cattle having BLV infection in 2020 (Mamanova et al., 2020; Polat et al., 2015). Limited veterinary resources and infrastructure in many regions make regular testing and disease monitoring difficult. Basic biosecurity practices, such as changing gloves and avoiding needle reuse, are not always followed. The introduction of new animals without knowing their BLV status is common, increasing the risk of spread within herds (Kuczewski et al., 2022). Cultural and management differences, such as communal grazing or uncontrolled breeding, also contribute to the spread of BLV (Kiugu, 2018). High-risk cattle are more likely to spread the virus, emphasizing the importance of monitoring and segregating them.
| Regions of Asia | Country | Area | Species | Sample Size | Sample | Test | Prevalence | Reference |
|---|---|---|---|---|---|---|---|---|
| Western Asia | United Arab Emirates | Abu Dhabi | Dairy cattle | 957 | Blood | ELISA | 25.7% overall | Hassan et al. (2020) |
| Turkey | - | Cattle | - | Serum | AGID | 21% overall | Burgu et al. (2005) | |
| Iraq | - | Cattle | 300 | Serum | Immunodiffusion test | 1.6% overall | Meas et al. (2003) | |
| Iraq | - | Cattle | 400 | Blood | PCR ELISA | 7% overall | Khudhair et al. (2016b) | |
| Central Asia | Kazakhstan | East Region | Cattle | - | Blood | PCR | 40.0–84.0% overall | Mamanova et al. (2020) |
| Eastern Asia | Japan | - | Dairy and Beef cattle | 20835 | Serum | ELISA | 35% overall, 40.9% Dairy cattle, 28.7% Beef cattle | Murakami et al. (2013) |
| Korea | - | Cattle | 4498 | Serum | ELISA | 10.20% overall | Kim et al. (2017) | |
| China | - | Cattle, Yak, Buffalo | - | Blood | ELISA, PCR, AGID | 10.00% overall | Ma et al. (2021) | |
| Taiwan | - | Dairy cattle | - | Serum | ELISA, PCR | 99.1% herd, 81.8% individual | Hsieh et al. (2019) | |
| Mongolia | Tuv, Arkhangai, Ulaanbaatar | Dairy cattle, native cattle, yaks | 517 | Blood | PCR | 3.9% overall | N. Ochirkhuu et al. (2016) | |
| Southern Asia | India | Himachal Pradesh | Dairy and Breeding cattle | 1511 | Blood | AGID | 9.09% overall | Katoch et al. (2017) |
| Iran | Isfahan and Bushehr provinces | Cattle, Sheep, Camel | 874 | Blood | Nested PCR | 22.1%-25.4% overall (5.3% Cattle, 22% Sheep, 0% Camel) | Nekoei et al. (2015) | |
| Pakistan | Northwest | Cattle | 600 | Blood | Indirect ELISA | 20% Cattle | Khan et al. (2020) | |
| Southeast Asia | Thailand | 9 provinces of Thailand | Cattle | 744 | Serum | ELISA, Nested PCR | 58.30% Cattle | Lee et al. (2016) |
| Philippines | - | Cattle | - | Blood | qPCR, Nested PCR | 23.1% Cattle | Polat et al. (2015) | |
| Myanmar | - | Cattle | - | Blood | qPCR | 37.04% Cattle | Mamanova et al. (2020) | |
| Cambodia | 5 provinces of Cambodia | Draught Cattle | 544 | Blood | ELISA | 5.3% Draught cattle | Meas et al. (2000) |
4. Pathogenesis and Clinical staging of BLV Infection
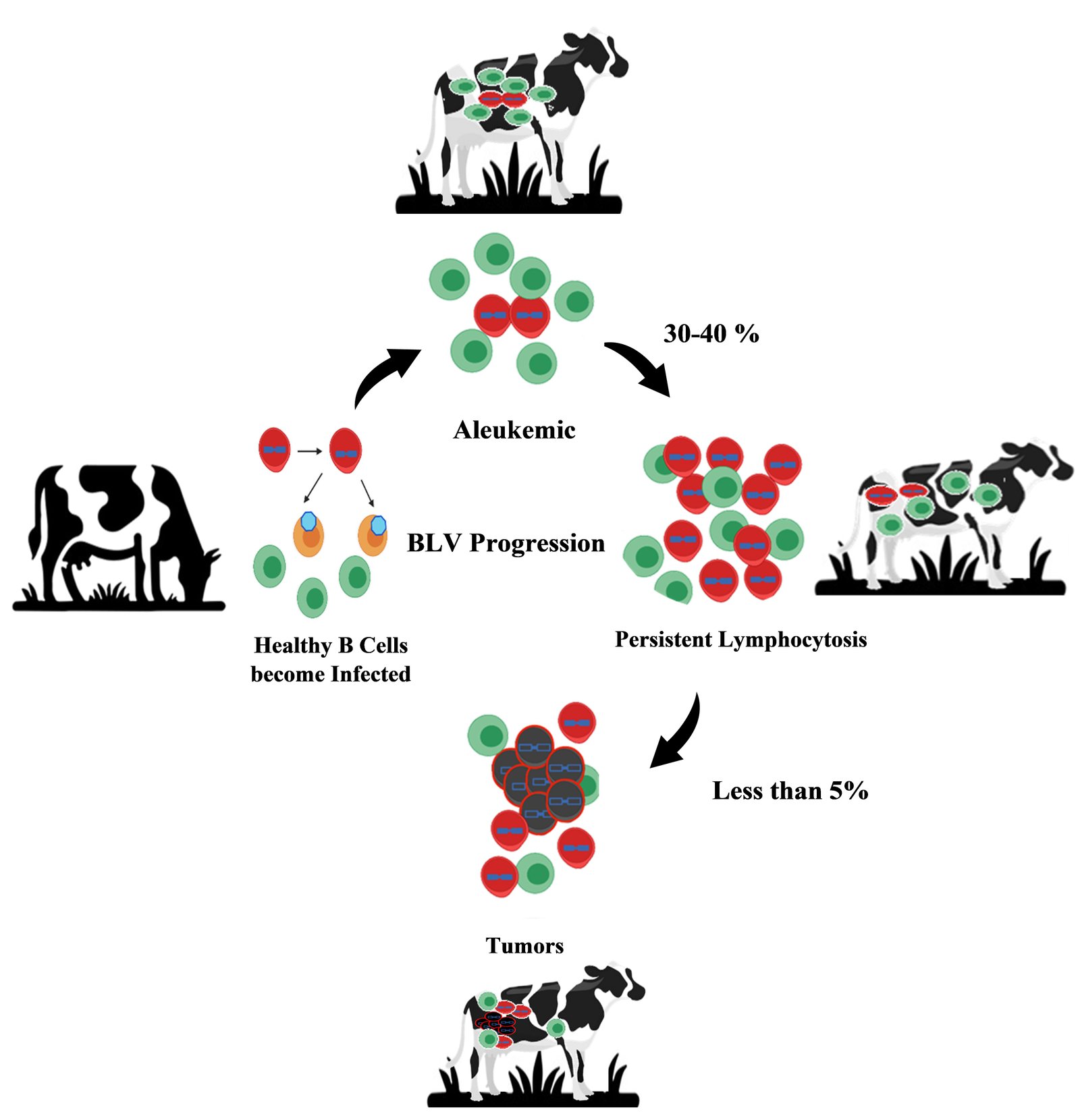
Bovine Leukemia Virus (BLV) specifically targets B-lymphocytes and enters these cells and integrates its proviral DNA into the host genome, enabling persistent infection. The viral envelope glycoproteins interact with specific surface receptors on B-lymphocytes to mediate viral entry. Toll-like receptors (TLRs), particularly TLR3, TLR7, and TLR9, are upregulated in infected animals, suggesting their involvement in viral recognition and entry mechanisms. Once inside the cell, the viral RNA genome is reverse-transcribed into DNA and integrated into the host genome. In veterinary medicine, clinical staging of EBL is a crucial procedure to evaluate the severity and progression of the disease in infected animals. Bovine leukemia is a fatal disorder characterized by neoplastic lymphocytosis and systemic lymphoma, with two types: EBL caused by BLV and SBL, not transmissible, and unknown etiology (Nishimori et al., 2017). The incubation period is key to understanding disease spread and progression. After a long incubation period of 7.0 years, 1.4% of EBL-infected dairy cattle developed lymphosarcoma (Tsutsui et al., 2016). Enzootic bovine leukosis manifests itself in distinct clinical stages based on serologic, hematologic, and clinical observations: asymptomatic, persistent lymphocytosis, and lymphosarcoma as described in Figure 1 (Radostits et al., 2006; Trono et al., 2001). Stage Ⅰ was characterized by seropositivity without any apparent clinical signs. Stage Ⅱ referred to as subclinical; there is an elevation in antibody levels but no observable clinical symptoms. Stage Ⅲ referred to as clinical, the disease becomes evident with visible clinical signs. Clinical signs include Lymphocytosis, swollen lymph nodes, progressive weight loss, decreased milk production, unilateral exophthalmos, and impaired fertility (Mekata et al., 2019; Santos et al., 2023). Finally, Stage Ⅳ represented the end-stage of the disease, where terminal lymphosarcomas or the extensive infiltrations of lymphoid tissue were observed (Burng, 1980; Radostits et al., 2006). About 60–70% of BLV-infected cattle were asymptomatic during the aleukemic stage, but after a latency that extends from a few months to years, 30% developed persistent lymphocytosis (PL), and 5% developed lymphoma, an incurable form of the disease. It mostly occurs in adult animals (Gillet et al., 2007). The vast majority of infected animals exhibited no clinical signs and remained carriers throughout life. All BLV-positive animals did not have lymphocytosis, which affected their health and spread infection through infected blood lymphocytes (Khudhair et al., 2016a; Trono et al., 2001).In cattle, a lymphocyte count exceeding 8000 lymphocytes/μL indicated persistent lymphocytosis (PL), a form of leukemic leukosis. A majority (70%) of BLV-positive cattle exhibited atypical lymphocytes (Khudhair et al., 2016b). BLV disrupts immune functioning and increases susceptibility to other infectious diseases (Emanuelson et al., 1992; Erskine et al., 2011). BLV prolongs immunosuppression during the periparturient period by increasing expression of immunological checkpoints in T cells, leading to higher rates of intramammary infections (do Nascimento et al., 2023). Studies have found a correlation between BLV infection and increased susceptibility to other common cattle diseases, particularly reproductive infectious diseases such as Bovine viral diarrhea, Bovine herpesvirus, and Leptospirosis (De Brun et al., 2023). BLV infection is associated with decreased milk production, shortened lifespan, and reduced immune response to immunization (De Brun et al., 2023). Persistent lymphocytosis in enzootic bovine leukosis involved elevated B-lymphocytes and B/T ratio inversion. PL was associated with immune disruptions and heightened vulnerability to infections, indicating its pre-tumoral nature in cattle (Okagawa et al., 2012; Suzuki et al., 2013). Animals remain persistently infected despite the absence of visible signs because it consistently triggers the immune response (Florins et al., 2007). Following infection, humoral and cytotoxic mediators impair the viral replication cycle, allowing only provirus-carrying cells to proliferate mitotically. Infected cells vigorously replicate, indicating a comparatively higher replication rate than normal to persist and multiply within the host (Elemans et al., 2014; Florins et al., 2007). Proviral DNA was found to be integrated a large number of genomic sites in both persistent lymphocytosis and lymph node tumor forms of bovine leukemia (Kettmann et al., 1980). Leisering found yellowish nodules with splenomegaly and tumors of B cell infiltrate in all body tissues like the liver, eyes, skin, lungs, and lymph nodes (Leisering, 1871). Prevalence varies, with ongoing research focusing on epidemiology, transmission, and control strategies to mitigate losses and potential public health concerns (Lv et al., 2024; Polat et al., 2015). BLV staging can differ between countries based on available resources and diagnostic tools. For example, countries with strong veterinary infrastructure may use advanced methods like proviral load testing, while others rely on basic clinical signs or ELISA due to limited resources. Treatment approaches also vary, with some countries focusing on culling and others emphasizing biosecurity and education.
5. BLV Genotypes Distribution in Asian Countries
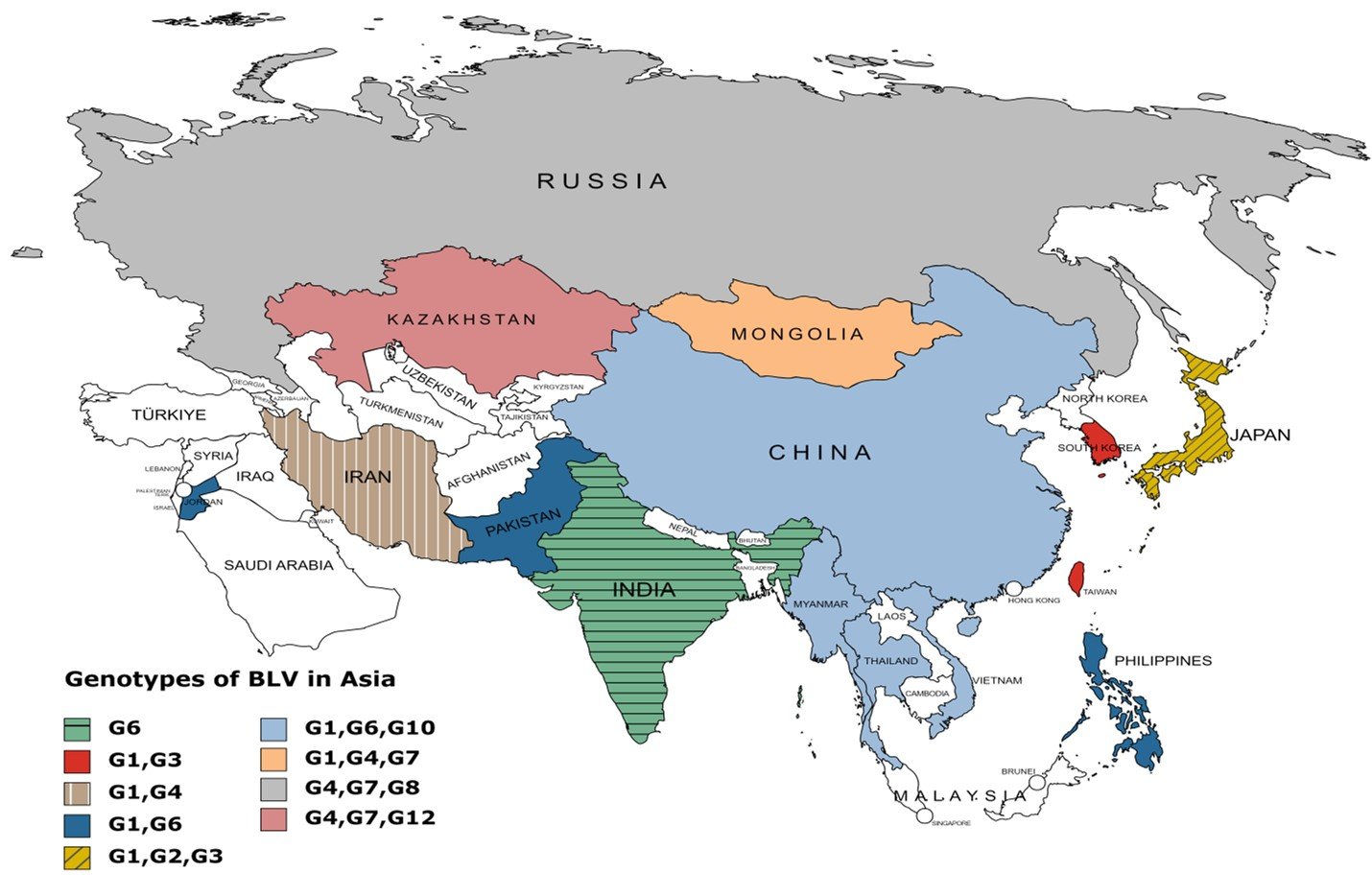
Bovine Leukemia Virus (BLV) genotypes exhibit mutations in the envelope (env) gene, which influence virulence and immune evasion. Notably, genotypes with changes in immunogenic epitopes are more adept at escaping host immune responses, potentially increasing transmission rates (Zhao et al., 2024). Specific mutations in the BLV genome, particularly in the long terminal repeat (LTR) and Tax regions, are linked to higher proviral loads, which correlate with increased virulence and transmissibility (Pluta et al., 2018). Strains with these mutations are more efficient in viral replication and immune evasion. BLV Genotype G1 is most predominant all over the world, including Asia, Europe, America, and Australia. In Asian countries, G1 is most dominant and continues to spread in all Asian countries having higher prevalence.G6 is the second most distributed genotype in Asian countries like Thailand, Colombia, Philippines, Iran, Japan, Taiwan, Turkey, Pakistan, Myanmar, Mongolia, Vietnam, China, South Korea, Jordan, and India.G4 is primarily distributed in Europe and American and Europe countries but now it is distributed in Iran, Mongolia, Russia, Turkey, and Kazakhstan. G2 is only found in Japan. G7 is commonly distributed in Russia, Kazakhstan and Mongolia. Another genotype, G10 mostly found in China, Vietnam, Myanmar, and Thailand. G12 is prevalent in Kazakhstan. These genotypes spread in Asia due to animal trading and exchange across national boundaries. The extensive dispersion of BLV genotypes across different regions, even those far apart, may be influenced by the viral transfer facilitated by the movement of live animal populations due to human migration and animal domestication (Reed, 1981).
The apparent BLV genotype distribution across Asia is displayed in Table 2 and Figure 2. In detail, Genotypes G1, G2, G3, G4, G6, G7, G8, G10, and G12 have been detected in all regions of Asia. Genotype G1, G2, and G3 in Japan (Matsumura et al., 2011); G4, G7, and G8 in Russia (Rola-Łuszczak et al., 2013); G1, G6, and G10 in Thailand (Lee et al., 2016); G1, G4, G7 in Mongolia (Nyamsuren Ochirkhuu et al., 2016); G4, G7, and G12 in Kazakhstan (Sultanov et al., 2022); G1 and G6 in Philippines, Jordan and Pakistan (Ababneh et al., 2012; Polat et al., 2015; Rola-Łuszczak et al., 2021); G1 and G4 in Iran and Turkey (Alkan et al., 2021; Degirmenci, 2011; Kazemimanesh et al., 2019); G1, G6 and G10 in Myanmar (Moe et al., 2020); G1, G6, and G10 in Vietnam (Le et al., 2020); G1 and G3 in Taiwan and South Korea (Hsieh et al., 2019; Lee et al., 2015); G6 in India have been investigated (Gautam et al., 2018). Analysis of Bovine leukemia virus's phylogenetic properties and ancestral traits indicates its progenitors originated in Asian regions, but further research is needed to determine its geographical origin. Keeping that in mind, Asia is the most susceptible and potential geographical region for BLV spread. These are commonly cattle species in Asia and are most susceptible to possessing, maintain, and transmitting the virus (Kohei Nishikaku et al., 2022).
| Regions of Asia | Country | Genotypes | Reference |
|---|---|---|---|
| Northern Asia | Russia | G4, G7, G8 | Rola-Łuszczak et al. (2013) |
| Western Asia | Turkey | G1, G4 | Alkan et al. (2021); Degirmenci (2011) |
| Colombia | G1, G6 | Corredor-Figueroa et al. (2020) | |
| Iran | G1, G4 | Kazemimanesh et al. (2019) | |
| Jordan | G1, G6 | Ababneh et al. (2012) | |
| Central Asia | Kazakhstan | G4, G7, G12 | Sultanov et al. (2022) |
| Eastern Asia | Japan | G1, G2, G3 | Matsumura et al. (2011) |
| China | G1, G6, G10 | Ababneh et al. (2012); Wang et al. (2018); Yu et al. (2019) | |
| Taiwan | G1, G3 | Hsieh et al. (2019) | |
| Mongolia | G1, G4, G7 | Nyamsuren Ochirkhuu et al. (2016) | |
| South Korea | G1, G3 | Lee et al. (2015) | |
| Southern Asia | India | G6 | Gautam et al. (2018) |
| Pakistan | G1, G6 | Rola-Łuszczak et al. (2021) | |
| Southeast Asia | Philippines | G1, G6 | Polat et al. (2015) |
| Thailand | G1, G6, G10 | Lee et al. (2016) | |
| Myanmar | G1, G6 | Moe et al. (2020) | |
| Vietnam | G1, G6, G10 | Le et al. (2020) |
6. Conclusion
There is a dearth of well-conducted studies on the prevalence and molecular characterization of enzootic bovine leukosis in Asia. This review provides the current status of bovine leukosis which is widely prevalent in Asian countries, and the majority of genotypes circulating in Asia are G1, G2, G3, G4, G6, G7, G8, G10, and G12. The prevalence level of BLV in different regions of Asia is quite variable in every region, relatively high prevelence shows the importance of highlighting this issue. Bovine leukosis is an important disease because it causes significant economic losses, and animals remain carriers throughout their lives. Therefore, the present review helps in the control of EBL by implementing a closed trading system in Asia to test and prevent the introduction of infected animals. Collaboration across countries may also play an important role in standardizing control measures and sharing resources, resulting in more effective and sustainable disease management.
Author contributions
All authors contributed significantly to the writing of this review paper. All authors have reviewed and agreed on the final version of the manuscript.
Acknowledgement
None
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
>Ababneh, M. M., Al-Rukibat, R. K., Hananeh, W. M., Nasar, A. T., & Al-Zghoul, M. B. (2012). Detection and molecular characterization of bovine leukemia viruses from Jordan. Arch Virol, 157(12), 2343-2348. https://doi.org/10.1007/s00705-012-1447-z
Abdala, A., Alvarez, I., Brossel, H., Calvinho, L., Carignano, H., Franco, L., Gazon, H., Gillissen, C., Hamaidia, M., & Hoyos, C. (2019). BLV: lessons on vaccine development. Retrovirology, 16(1), 1-6. https://doi.org/10.1186/s12977-019-0488-8
Alkan, F., Karayel-Hacioglu, I., Duran Yelken, S., & Coskun, N. (2021). The genotype determination and molecular characterization of bovine leukemia virus in Turkey. Veterinarski Arhiv, 91(3), 237-247. https://doi.org/10.24099/vet.arhiv.1214
Amirpour Haredasht, S., Vidal, G., Edmondson, A., Moore, D., Silva-del-Río, N., & Martínez-López, B. (2018). Characterization of the temporal trends in the rate of cattle carcass condemnations in the US and dynamic modeling of the condemnation reasons in California with a seasonal component. Frontiers in veterinary science, 5, 87. https://doi.org/10.3389/fvets.2018.00087
Barez, P. Y., de Brogniez, A., Carpentier, A., Gazon, H., Gillet, N., Gutierrez, G., Hamaidia, M., Jacques, J. R., Perike, S., Neelature Sriramareddy, S., Renotte, N., Staumont, B., Reichert, M., Trono, K., & Willems, L. (2015). Recent Advances in BLV Research. Viruses, 7(11), 6080-6088. https://doi.org/10.3390/v7112929
Bartlett, P. C., Norby, B., Byrem, T. M., Parmelee, A., Ledergerber, J. T., & Erskine, R. J. (2013). Bovine leukemia virus and cow longevity in Michigan dairy herds. J Dairy Sci, 96(3), 1591-1597. https://doi.org/10.3168/jds.2012-5930
Burgu, I., Alkan, F., Karaoglu, T., Bilge-Dagalp, S., Can-Sahna, K., Gungor, B., & Demir, B. (2005). Control and eradication programme of enzootic bovine leucosis (EBL) from selected dairy herds in Turkey. Dtsch Tierarztl Wochenschr, 112(7), 271-274. https://www.ncbi.nlm.nih.gov/pubmed/16124702
Burng, A. (1980). Bovine leukemia virus: molecular biology and epidemiology. Viral oncology, 231-289.
Corredor-Figueroa, A. P., Salas, S., Olaya-Galan, N. N., Quintero, J. S., Fajardo, A., Sonora, M., Moreno, P., Cristina, J., Sanchez, A., Tobon, J., Ortiz, D., & Gutierrez, M. F. (2020). Prevalence and molecular epidemiology of bovine leukemia virus in Colombian cattle. Infect Genet Evol, 80, 104171. https://doi.org/10.1016/j.meegid.2020.104171
Da, Y., Shanks, R. D., Stewart, J. A., & Lewin, H. A. (1993). Milk and fat yields decline in bovine leukemia virus-infected Holstein cattle with persistent lymphocytosis. Proc Natl Acad Sci U S A, 90(14), 6538-6541. https://doi.org/10.1073/pnas.90.14.6538
De Brun, L., Mionetto, M., Rodríguez, F., Brandl, S., Hubner, S., Fischer, G., & Puentes, R. (2023). Natural association between bovine leukemia virus and reproductive infectious diseases. Acta Scientiae Veterinariae, 51. 10.22456/1679-9216.129251
Degirmenci, H. (2011). Detection of bovine leukemia virus by using serological and molecular methods in Marmara region PhD Thesis, Istanbul University, Istanbul, Turkey].
do Nascimento, A. M. M., de Souza, C. M. S., Oliveira, A. C. D., Blagitz, M. G., Sanchez, E. M. R., Della Libera, A. M. M. P., Leite, R. d. M. H., de Carvalho Fernandes, A. C., Souza, F. N. J. V. I., & Immunopathology. (2023). The bovine leukemia virus infection prolongs immunosuppression in dairy cows during the periparturient period by sustaining higher expression of immunological checkpoints in T cells. Vet Immunol Immunopathol, 263, 110636. https://doi.org/10.1016/j.vetimm.2023.110636
Elemans, M., Florins, A., Willems, L., & Asquith, B. (2014). Rates of CTL killing in persistent viral infection in vivo. PLoS Comput Biol, 10(4), e1003534. https://doi.org/10.1371/journal.pcbi.1003534
Emanuelson, U., Scherling, K., & Pettersson, H. (1992). Relationships between herd bovine leukemia virus infection status and reproduction, disease incidence, and productivity in Swedish dairy herds. Preventive veterinary medicine, 12(1-2), 121-131. https://doi.org/10.1016/0167-5877(92)90075-q
Erskine, R., Bartlett, P., Byrem, T., Render, C., Febvay, C., & Houseman. (2012). Association between bovine leukemia virus, production, and population age in Michigan dairy herds. Journal of Dairy Science, 95(2), 727-734. https://doi.org/10.3168/jds.2011-4760
Erskine, R. J., Bartlett, P. C., Sabo, K. M., & Sordillo, L. M. (2011). Bovine Leukemia Virus Infection in Dairy Cattle: Effect on Serological Response to Immunization against J5 Escherichia coli Bacterin. Vet Med Int, 2011, 915747. https://doi.org/10.4061/2011/915747
Feliziani, F., Martucciello, A., Iscaro, C., Vecchio, D., Petrini, S., Grassi, C., Bazzucchi, M., & De Carlo, E. (2017). Bovine leukemia virus: Experimental infection in buffaloes and evaluation of diagnostic test reliability. Res Vet Sci, 114, 450-454. https://doi.org/10.1016/j.rvsc.2017.07.021
Florins, A., Boxus, M., Vandermeers, F., Verlaeten, O., Bouzar, A. B., Defoiche, J., Hubaux, R., Burny, A., Kettmann, R., & Willems, L. (2008). Emphasis on cell turnover in two hosts infected by bovine leukemia virus: a rationale for host susceptibility to disease. Vet Immunol Immunopathol, 125(1-2), 1-7. https://doi.org/10.1016/j.vetimm.2008.04.007
Florins, A., Gillet, N., Asquith, B., Boxus, M., Burteau, C., Twizere, J. C., Urbain, P., Vandermeers, F., Debacq, C., Sanchez-Alcaraz, M. T., Schwartz-Cornil, I., Kerkhofs, P., Jean, G., Thewis, A., Hay, J., Mortreux, F., Wattel, E., Reichert, M., Burny, A.,…Willems, L. (2007). Cell dynamics and immune response to BLV infection: a unifying model. Front Biosci, 12, 1520-1531. https://doi.org/10.2741/2165
Gautam, S., Mishra, N., Kalaiyarasu, S., Jhade, S. K., & Sood, R. (2018). Molecular Characterization of Bovine Leukaemia Virus (BLV) Strains Reveals Existence of Genotype 6 in Cattle in India with evidence of a new subgenotype. Transbound Emerg Dis, 65(6), 1968-1978. https://doi.org/10.1111/tbed.12979
Gillet, N., Florins, A., Boxus, M., Burteau, C., Nigro, A., Vandermeers, F., Balon, H., Bouzar, A. B., Defoiche, J., Burny, A., Reichert, M., Kettmann, R., & Willems, L. (2007). Mechanisms of leukemogenesis induced by bovine leukemia virus: prospects for novel anti-retroviral therapies in human. Retrovirology, 4(1), 18. https://doi.org/10.1186/1742-4690-4-18
Hassan, N. A. D., Mohteshamuddin, K., Anthony, A., Al Aiyan, A., Mohamed, M. E. H., Abdalla Alfaki, I. M., & Barigye, R. (2020). Serological evidence of enzootic bovine leukosis in the periurban dairy cattle production system of Al Ain, United Arab Emirates. Trop Anim Health Prod, 52(5), 2327-2332. https://doi.org/10.1007/s11250-020-02262-1
Hsieh, J. C., Li, C. Y., Hsu, W. L., & Chuang, S. T. (2019). Molecular Epidemiological and Serological Studies of Bovine Leukemia Virus in Taiwan Dairy Cattle. Front Vet Sci, 6, 427. https://doi.org/10.3389/fvets.2019.00427
Joris, T., Safari, R., Jacques, J.-R., & Willems, L. (2021). Bovine leukemia virus (Retroviridae). https://doi.org/10.1016/b978-0-12-814515-9.00059-x
Juliarena, M. A., Barrios, C. N., Lützelschwab, C. M., Esteban, E. N., & Gutierrez, S. E. (2017). Bovine leukemia virus: current perspectives. Virus Adaptation and Treatment. https://doi.org/10.2147/VAAT.S113947
Katoch, S., Dohru, S., Sharma, M., Vashist, V., Chahota, R., Dhar, P., Thakur, A., & Verma, S. (2017). Seroprevalence of viral and bacterial diseases among the bovines in Himachal Pradesh, India. Veterinary World, 10(12), 1421. https://doi.org/10.14202/vetworld.2017.1421-1426
Kazemimanesh, M., Madadgar, O., Steinbach, F., Choudhury, B., & Azadmanesh, K. (2019). Detection and molecular characterization of bovine leukemia virus in various regions of Iran. Journal of General Virology, 100(9), 1315-1327. https://doi.org/10.1099/jgv.0.001303
Kettmann, R., Cleuter, Y., Mammerickx, M., Meunier-Rotival, M., Bernardi, G., Burny, A., & Chantrenne, H. (1980). Genomic integration of bovine leukemia provirus: comparison of persistent lymphocytosis with lymph node tumor form of enzootic. Proc Natl Acad Sci U S A, 77(5), 2577-2581. https://doi.org/10.1073/pnas.77.5.2577
Khan, M. F., Siddique, U., Shah, A. A., Khan, I., Anwar, F., Ahmad, I., Zeb, M. T., Hassan, M. F., & Ali, T. (2020). Seroprevalence of bovine leukemia virus (BLV) in cattle from the north west of Pakistan. Pakistan Veterinary Journal. https://doi.org/10.29261/pakvetj/2019.103
Khudhair, Y. I., Hasso, S. A., Yaseen, N. Y., & Al-Shammari, A. M. (2016a). Serological and molecular detection of bovine leukemia virus in cattle in Iraq. Emerg Microbes Infect, 5(6), e56. https://doi.org/10.1038/emi.2016.60
Khudhair, Y. I., Hasso, S. A., Yaseen, N. Y., & Al-Shammari, A. M. (2016b). Serological and molecular detection of bovine leukemia virus in cattle in Iraq. Emerg. microbes & infect., 5(6), e56. https://doi.org/10.1038/emi.2016.60
Kim, H.-U., Lee, E.-Y., Lee, K.-K., Kim, S.-H., Moon, B.-Y., So, B.-J., & Kim, Y.-H. (2017). Seroprevalence of the bovine leukemia virus among Korean native cattle in South Korea. Preventive veterinary medicine, 41(1), 52-55. https://doi.org/10.14202/vetworld.2024.1715-1721
Kiugu, E. K. (2018). Seroprevalence of bovine leukosis infection in selected farming systems in Kenya http://hdl.handle.net/11295/104163
Kuczewski, A., Adams, C., Lashewicz, B., & van der Meer, F. (2022). Alberta dairy farmers' and veterinarians' opinion about bovine leukemia virus control measures. Prev Vet Med, 200, 105590. https://doi.org/10.1016/j.prevetmed.2022.105590
Le, D. T., Yamashita-Kawanishi, N., Okamoto, M., Nguyen, S. V., Nguyen, N. H., Sugiura, K., Miura, T., & Haga, T. (2020). Detection and genotyping of bovine leukemia virus (BLV) in Vietnamese cattle. J Vet Med Sci, 82(7), 1042-1050. https://doi.org/10.1292/jvms.20-0094
Lee, E., Kim, E. J., Joung, H. K., Kim, B. H., Song, J. Y., Cho, I. S., Lee, K. K., & Shin, Y. K. (2015). Sequencing and phylogenetic analysis of the gp51 gene from Korean bovine leukemia virus isolates. Virol J, 12, 64. https://doi.org/10.1186/s12985-015-0286-4
Lee, E., Kim, E. J., Ratthanophart, J., Vitoonpong, R., Kim, B. H., Cho, I. S., Song, J. Y., Lee, K. K., & Shin, Y. K. (2016). Molecular epidemiological and serological studies of bovine leukemia virus (BLV) infection in Thailand cattle. Infect Genet Evol, 41, 245-254. https://doi.org/10.1016/j.meegid.2016.04.010
Leisering, A. (1871). Hypertrophy der Malpigischen Korperchen der Milz. Bericht uber das Veterinarwesen im Konigreich Sachsen, 16, 15-16.
Lv, G., Wang, J., Lian, S., Wang, H., & Wu, R. (2024). The Global Epidemiology of Bovine Leukemia Virus: Current Trends and Future Implications. Animals (Basel), 14(2), 297. https://doi.org/10.3390/ani14020297
Ma, B.-Y., Gong, Q.-L., Sheng, C.-Y., Liu, Y., Ge, G.-Y., Li, D.-L., Diao, N.-C., Shi, K., Li, J.-M., & Sun, Z.-B. (2021). Prevalence of bovine leukemia in 1983–2019 in China: A systematic review and meta-analysis. Microbial pathogenesis, 150, 104681. https://doi.org/10.1016/j.micpath.2020.104681
Mamanova, S., Kalisynov, B., Saduakasova, M., Bashenova, E., & Maukish, A. (2020). Analysis of the epizootic situation on bovine leukemia for 2015–2019 in the East Kazakhstan region. Collect. KazSRVI, 66, 60-64.
Marawan, M. A., Alouffi, A., El Tokhy, S., Badawy, S., Shirani, I., Dawood, A., Guo, A., Almutairi, M. M., Alshammari, F. A., & Selim, A. (2021). Bovine Leukaemia Virus: Current Epidemiological Circumstance and Future Prospective. Viruses, 13(11), 2167. https://doi.org/10.3390/v13112167
Matsumura, K., Inoue, E., Osawa, Y., & Okazaki, K. (2011). Molecular epidemiology of bovine leukemia virus associated with enzootic bovine leukosis in Japan. Virus Res, 155(1), 343-348. https://doi.org/10.1016/j.virusres.2010.11.005
Meas, S., Ohashi, K., Tum, S., Chhin, M., Te, K., Miura, K., Sugimoto, C., & Onuma, M. (2000). Seroprevalence of bovine immunodeficiency virus and bovine leukemia virus in draught animals in Cambodia. J Vet Med Sci, 62(7), 779-781. https://doi.org/10.1292/jvms.62.779
Meas, S., Yilmaz, Z., Usui, T., Torun, S., Yesilbag, K., Ohashi, K., & Onuma, M. (2003). Evidence of bovine immunodeficiency virus in cattle in Turkey. Jpn J Vet Res, 51(1), 3-8. https://doi.org/10.14943/jjvr.51.1.3
Mekata, H., Sekiguchi, S., Konnai, S., & Kirino, Y. (2019). Current prevalence and risk factors associated with bovine leukemia virus infection in Japanese dairy farms. Journal of veterinary Medical Science, 81(9).
Moe, K. K., Polat, M., Borjigin, L., Matsuura, R., Hein, S. T., Moe, H. H., & Aida, Y. (2020). New evidence of bovine leukemia virus circulating in Myanmar cattle through epidemiological and molecular characterization. PloS one, 15(2), e0229126. https://doi.org/10.1371/journal.pone.0229126
Molnar, E., Molnar, L., Guedes, V. T., & de Lima, E. S. (2000). Naturally occurring bovine leukosis virus in water buffalo (Bubalus bubalis) in Brazil. Vet Rec, 146(24), 705-706. https://doi.org/10.1136/vr.146.24.705
Mousavi, S., Haghparast, A., Mohammadi, G., & Tabatabaeizadeh, S.-E. (2014). Prevalence of bovine leukemia virus (BLV) infection in the northeast of Iran. Veterinary research forum: an international quarterly journal,
Murakami, K., Kobayashi, S., Konishi, M., Kameyama, K.-i., & Tsutsui, T. (2013). Nationwide survey of bovine leukemia virus infection among dairy and beef breeding cattle in Japan from 2009–2011. Journal of veterinary Medical Science, 75(8), 1123-1126. https://doi.org/10.1292/jvms.12-0374
Nakada, S., Fujimoto, Y., Kohara, J., Adachi, Y., & Makita, K. (2022). Estimation of economic loss by carcass weight reduction of Japanese dairy cows due to infection with bovine leukemia virus. Preventive veterinary medicine, 198, 105528. https://doi.org/10.1016/j.prevetmed.2021.105528
Nakada, S., Fujimoto, Y., Kohara, J., & Makita, K. (2023). Economic losses associated with mastitis due to bovine leukemia virus infection. Journal of Dairy Science, 106(1), 576-588. https://doi.org/10.3168/jds.2021-21722
Nekoei, S., Hafshejani, T. T., Doosti, A., & Khamesipour, F. (2015). Molecular detection of bovine leukemia virus in peripheral blood of Iranian cattle, camel and sheep. Pol J Vet Sci, 18(4), 703-707. https://doi.org/10.1515/pjvs-2015-0091
Nekouei, O., VanLeeuwen, J., Stryhn, H., Kelton, D., & Keefe, G. (2016). Lifetime effects of infection with bovine leukemia virus on longevity and milk production of dairy cows. Prev Vet Med, 133, 1-9. https://doi.org/10.1016/j.prevetmed.2016.09.011
Nishikaku, K., Nishibori, M., Imakawa, K., & Kobayashi, T. (2022). Phylogenomics and spatiotemporal dynamics of bovine leukemia virus focusing on Asian native cattle: insights into the early origin and global dissemination. Frontiers of Microbiology, 13, 917324. https://doi.org/10.3389/fmicb.2022.917324
Nishikaku, K., Noguchi, T., Murakami, S., Torii, Y., & Kobayashi, T. (2022). Molecular analysis of bovine leukemia virus in early epidemic phase in Japan using archived formalin fixed paraffin embedded histopathological specimens. J Vet Med Sci, 84(3), 350-357. https://doi.org/10.1292/jvms.21-0570
Nishimori, A., Konnai, S., Okagawa, T., Maekawa, N., Goto, S., Ikebuchi, R., Nakahara, A., Chiba, Y., Ikeda, M., & Murata, S. (2017). Identification of an atypical enzootic bovine leukosis in Japan by using a novel classification of bovine leukemia based on immunophenotypic analysis. Clinical and Vaccine Immunology, 24(9), e00067-00017. https://doi.org/10.1128/CVI.00067-17
Norby, B., Bartlett, P. C., Byrem, T. M., & Erskine, R. J. (2016). Effect of infection with bovine leukemia virus on milk production in Michigan dairy cows. J Dairy Sci, 99(3), 2043-2052. https://doi.org/10.3168/jds.2015-10089
Ochirkhuu, N., Konnai, S., Odbileg, R., Nishimori, A., Okagawa, T., Murata, S., & Ohashi, K. (2016). Detection of bovine leukemia virus and identification of its genotype in Mongolian cattle. Arch Virol, 161(4), 985-991. https://doi.org/10.1007/s00705-015-2676-8
Ochirkhuu, N., Konnai, S., Odbileg, R., Nishimori, A., Okagawa, T., Murata, S., & Ohashi, K. (2016). Detection of bovine leukemia virus and identification of its genotype in Mongolian cattle. Arch. Virol., 161(4), 985-991.
Okagawa, T., Konnai, S., Ikebuchi, R., Suzuki, S., Shirai, T., Sunden, Y., Onuma, M., Murata, S., & Ohashi, K. (2012). Increased bovine Tim-3 and its ligand expressions during bovine leukemia virus infection. Vet Res, 43(1), 45. https://doi.org/10.1186/1297-9716-43-45
Olaya-Galán, N. N., Corredor-Figueroa, A. P., Velandia-Álvarez, S., Vargas-Bermudez, D. S., Fonseca-Ahumada, N., Nuñez, K., Jaime, J., & Gutiérrez, M. F. (2022). Evidence of bovine leukemia virus circulating in sheep and buffaloes in Colombia: insights into multispecies infection. Archives of Virology, 167(3), 807-817. https://doi.org/10.1007/s00705-021-05285-7
Ott, S., Johnson, R., & Wells, S. (2003). Association between bovine-leukosis virus seroprevalence and herd-level productivity on US dairy farms. Preventive veterinary medicine, 61(4), 249-262. https://doi.org/10.1016/j.prevetmed.2003.08.003
Pluta, A., Rola-Łuszczak, M., Douville, R. N., & Kuźmak, J. (2018). Bovine leukemia virus long terminal repeat variability: identification of single nucleotide polymorphisms in regulatory sequences. Virology Journal, 15, 1-14. https://doi.org/10.1186/s12985-018-1062-z
Polat, M., Ohno, A., Takeshima, S. N., Kim, J., Kikuya, M., Matsumoto, Y., Mingala, C. N., Onuma, M., & Aida, Y. (2015). Detection and molecular characterization of bovine leukemia virus in Philippine cattle. Arch Virol, 160(1), 285-296. https://doi.org/10.1007/s00705-014-2280-3
Quadros, D. L., Puhl, K., Ribeiro, V. A., Frandoloso, R., & Kreutz, L. C. (2024). The transmission of bovine leukemia virus to calves occurs mostly through colostrum and milk. Veterinary World, 17(12), 2918. https://doi.org/10.14202/vetworld.2024.2918-2924
Radostits, O. M., Gay, C., Hinchcliff, K. W., & Constable, P. D. (2006). Veterinary Medicine E-Book: A textbook of the diseases of cattle, horses, sheep, pigs and goats. Elsevier Health Sciences.
Reed, V. I. (1981). Enzootic bovine leukosis. Can Vet J, 22(4), 95-102. https://www.ncbi.nlm.nih.gov/pubmed/6265053
Rodríguez, S. M., Florins, A., Gillet, N., De Brogniez, A., Sánchez-Alcaraz, M. T., Boxus, M., Boulanger, F., Gutiérrez, G., Trono, K., & Alvarez, I. J. V. (2011). Preventive and therapeutic strategies for bovine leukemia virus: lessons for HTLV. 3(7), 1210.
Rola-Łuszczak, M., Pluta, A., Olech, M., Donnik, I., Petropavlovskiy, M., Gerilovych, A., Vinogradova, I., Choudhury, B., & Kuźmak, J. (2013). The molecular characterization of bovine leukaemia virus isolates from Eastern Europe and Siberia and its impact on phylogeny. PloS one, 8(3), e58705. https://doi.org/10.1371/journal.pone.0058705
Rola-Łuszczak, M., Sakhawat, A., Pluta, A., Ryło, A., Bomba, A., Bibi, N., & Kuźmak, J. (2021). Molecular characterization of the env gene of bovine leukemia virus in cattle from Pakistan with NGS-based evidence of virus heterogeneity. pathogens, 10(7), 910. https://doi.org/10.3390/pathogens10070910
Romero, C., Aguiar, A., Zanocchi, H., Abaracon, D., & Rowe, C. (1981). Susceptibility of the water buffalo (Bubalis bubalis) to enzootic bovine leukosis virus. Pesquisa Veterinaria Brasileria.
Sajiki, Y., Konnai, S., Nishimori, A., Okagawa, T., Maekawa, N., Goto, S., Nagano, M., Kohara, J., Kitano, N., Takahashi, T., Tajima, M., Mekata, H., Horii, Y., Murata, S., & Ohashi, K. (2017). Intrauterine infection with bovine leukemia virus in pregnant dam with high viral load. J Vet Med Sci, 79(12), 2036-2039. https://doi.org/10.1292/jvms.17-0391
Samad, A., Meghla, N. S., Nain, Z., Karpinski, T. M., & Rahman, M. S. (2022). Immune epitopes identification and designing of a multi-epitope vaccine against bovine leukemia virus: a molecular dynamics and immune simulation approaches. Cancer Immunol Immunother, 71(10), 2535-2548. https://doi.org/10.1007/s00262-022-03181-w
Santos, I. R., Henker, L. C., Kemper, R. T., Bertolini, M., Driemeier, D., & Pavarini, S. P. (2023). Enzootic bovine leukosis in a cow. Brazillian Journal of Veterinary Pathology, 16(1), 71-77.
Shrestha, S., Orsel, K., Barkema, H. W., Martins, L., Shrestha, S., & van der Meer, F. (2024). Effects of bovine leukemia virus seropositivity and proviral load on milk, fat, and protein production of dairy cows. J Dairy Sci, 107(1), 530-539. https://doi.org/10.3168/jds.2023-23695
Suarez Archilla, G., Gutierrez, G., Camussone, C., Calvinho, L., Abdala, A., Alvarez, I., Petersen, M., Franco, L., Destefano, G., Monti, G., Jacques, J. R., Joris, T., Willems, L., & Trono, K. (2022). A safe and effective vaccine against bovine leukemia virus. Front Immunol, 13, 980514. https://doi.org/10.3389/fimmu.2022.980514
Sultanov, A., Rola-Luszczak, M., Mamanova, S., Rylo, A., Osinski, Z., Saduakassova, M. A., Bashenova, E., & Kuzmak, J. (2022). Molecular Characterization of Bovine Leukemia Virus with the Evidence of a New Genotype Circulating in Cattle from Kazakhstan. pathogens, 11(2), 180. https://doi.org/10.3390/pathogens11020180
Suzuki, S., Konnai, S., Okagawa, T., Ikebuchi, R., Shirai, T., Sunden, Y., Mingala, C. N., Murata, S., & Ohashi, K. (2013). Expression analysis of Foxp3 in T cells from bovine leukemia virus infected cattle. Microbiol Immunol, 57(8), 600-604. https://doi.org/10.1111/1348-0421.12073
Taxis, T. M., Harbowy, R. M., Niles, D., Sporer, K. R. B., & Bartlett, P. C. (2023). Controlling bovine leukemia virus in a large dairy herd by selective culling based on diagnostic testing. Applied Animal Science, 39(2), 40-43. https://doi.org/10.15232/aas.2022-02347
Trono, K. G., Perez-Filgueira, D. M., Duffy, S., Borca, M. V., & Carrillo, C. (2001). Seroprevalence of bovine leukemia virus in dairy cattle in Argentina: comparison of sensitivity and specificity of different detection methods. Vet Microbiol, 83(3), 235-248. https://doi.org/10.1016/s0378-1135(01)00420-5
Tsutsui, T., Kobayashi, S., Hayama, Y., & Yamamoto, T. (2016). Fraction of bovine leukemia virus-infected dairy cattle developing enzootic bovine leukosis. Prev Vet Med, 124, 96-101. https://doi.org/10.1016/j.prevetmed.2015.11.019
Wang, M., Wang, Y., Baloch, A. R., Pan, Y., Xu, F., Tian, L., & Zeng, Q. (2018). Molecular epidemiology and characterization of bovine leukemia virus in domestic yaks (Bos grunniens) on the Qinghai-Tibet Plateau, China. Arch Virol, 163(3), 659-670. https://doi.org/10.1007/s00705-017-3658-9
White, T. L., & Moore, D. A. (2009). Reasons for whole carcass condemnations of cattle in the United States and implications for producer education and veterinary intervention. J Am Vet Med Assoc, 235(8), 937-941. https://doi.org/10.2460/javma.235.8.937
Yu, C., Wang, X., Zhou, Y., Wang, Y., Zhang, X., & Zheng, Y. (2019). Genotyping bovine leukemia virus in dairy cattle of Heilongjiang, northeastern China. BMC Vet Res, 15(1), 179. https://doi.org/10.1186/s12917-019-1863-3
Zhao, Y., Zhu, X., Zhang, Z., Chen, J., Chen, Y., Hu, C., Chen, X., Robertson, I. D., & Guo, A. (2024). The Prevalence and Molecular Characterization of Bovine Leukemia Virus among Dairy Cattle in Henan Province, China. Viruses, 16(9), 1399. https://doi.org/10.3390/v16091399