Parasites as drivers of host diversification, including domestication
DOI:
https://doi.org/10.71320/bcs.0015Keywords:
Parasitism, Domestication, Diversification, EvolutionAbstract
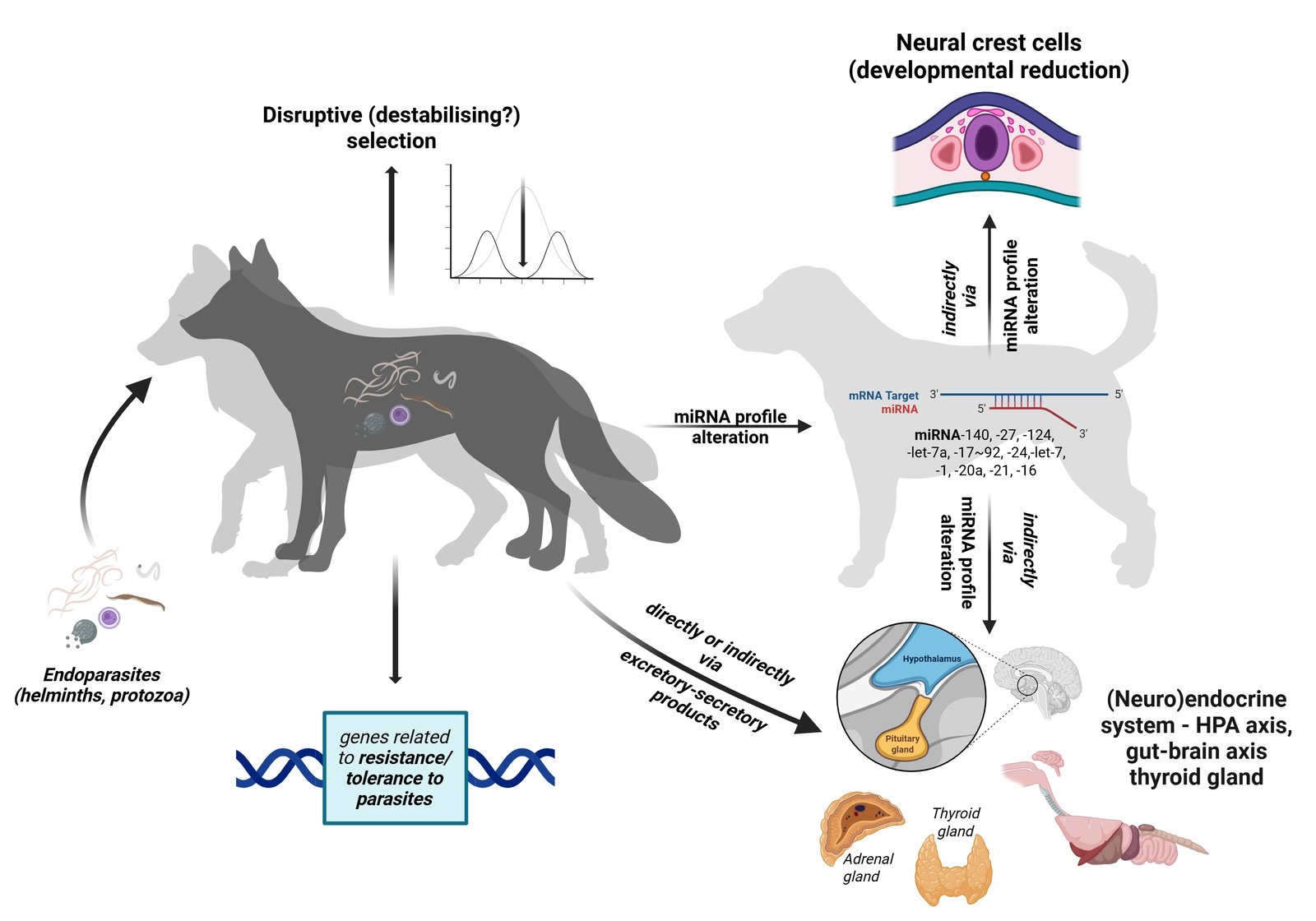
Parasites are ubiquitous evolutionary agents that, among other things, drive the diversification of hosts. This could also apply to animal domestication, one of the most striking evolutionary processes generating phenotypic diversity, which has recently been proposed as a parasite-mediated process (“The parasites-mediated domestication hypothesis”, PMD). The PMD is based on the fact that parasites can alter the (neuro)endocrine system (hypothalamic-pituitary-adrenal (HPA) axis, gut-brain axis, thyroid secretion, etc.), influence gene expression and ontogenetic systems, including neural crest cell development, and thus modulate physiological, behavioural and developmental components underlying the domestication. In this article, domestication is therefore placed in the context of parasite-driven diversification. The rationale for the parasite-mediated domestication process and associated diversification is supported by explanations of possible underlying mechanisms in light of the pioneering work of the Belyaev fox experiment and the later proposed hypothesis on the role of neural crest cells. Finally, a framework for plausible comparative and experimental approaches to test the PMD is proposed. Placing domestication in the context of the parasite-driven host diversification process provides a broad perspective for future research in the field of animal evolution and domestication.
References
Adamo, S. A. (2013). Parasites: evolution’s neurobiologists. Journal of Experimental Biology, 216(1), 3-10. https://doi.org/10.1242/jeb.073601 DOI: https://doi.org/10.1242/jeb.073601
Aebischer, T., Matuschewski, K., & Hartmann, S. (2018). Parasite infections: from experimental models to natural systems. Frontiers in Cellular and Infection Microbiology, 8, 12. https://doi.org/10.3389/fcimb.2018.00012 DOI: https://doi.org/10.3389/fcimb.2018.00012
Antonaci, M., & Wheeler, G. N. (2022). MicroRNAs in neural crest development and neurocristopathies. Biochemical Society Transactions, 50(2), 965-974. https://doi.org/10.1042/BST20210828 DOI: https://doi.org/10.1042/BST20210828
Bagnaresi, P., Nakabashi, M., Thomas, A. P., Reiter, R. J., & Garcia, C. R. (2012). The role of melatonin in parasite biology. Molecular and Biochemical Parasitology, 181, 1-6. https://doi.org/10.1016/j.molbiopara.2011.09.010 DOI: https://doi.org/10.1016/j.molbiopara.2011.09.010
Barribeau, S. M., Sadd, B. M., du Plessis, L., & Schmid-Hempel, P. (2014). Gene expression differences underlying genotype-by-genotype specificity in a host–parasite system. Proceedings of the National Academy of Sciences, 111(9), 3496-3501. https://doi.org/10.1073/pnas.1318628111 DOI: https://doi.org/10.1073/pnas.1318628111
Belyaev, D. K. (1969). Domestication of animals. Science Journal 5, 47-52.
Belyaev, D. K. (1979). Destabilizing selection as a factor in domestication. Journal of Heredity, 70, 301-308. https://doi.org/10.1093/oxfordjournals.jhered.a109263 DOI: https://doi.org/10.1093/oxfordjournals.jhered.a109263
Blanchet, S., Rey, O., Berthier, P., Lek, S., & Loot, G. (2009). Evidence of parasite‐mediated disruptive selection on genetic diversity in a wild fish population. Molecular Ecology, 18, 1112-1123. https://doi.org/10.1111/j.1365-294X.2009.04099.x DOI: https://doi.org/10.1111/j.1365-294X.2009.04099.x
Blecharz-Klin, K., Świerczyńska, M., Piechal, A., Wawer, A., Joniec-Maciejak, I., Pyrzanowska J, Wojnar, E., Zawistowska-Deniziak, A., Sulima-Celińska, A., Młocicki, D., & Mirowska-Guzel, D. (2022). Infection with intestinal helminth (Hymenolepis diminuta) impacts exploratory behavior and cognitive processes in rats by changing the central level of neurotransmitters. PLoS Pathogens, 18(3), e1010330. https://doi.org/10.1371/journal.ppat.1010330 DOI: https://doi.org/10.1371/journal.ppat.1010330
Breyner, N. M., Hecht, M., Nitz, N., Rose, E., & Carvalho, J. L. (2020). In vitro models for investigation of the host-parasite interface-possible applications in acute Chagas disease. Acta tropica, 202, 105262. https://doi.org/10.1016/j.actatropica.2019.105262 DOI: https://doi.org/10.1016/j.actatropica.2019.105262
Buck, A. H., Coakley, G., Simbari, F., McSorley, H. J., Quintana, J. F., Le Bihan, T., Kumar, S., Abreu-Goodger, C., Lear, M., Harcus, Y., Ceroni, A., Babayan, S. A., Blaxter, M., Ivens, A., & Maizels, R. M. (2014). Exosomes secreted by nematode parasites transfer small RNAs to mammalian cells and modulate innate immunity. Nature communications, 5(1), 5488. https://doi.org/10.1038/ncomms6488 DOI: https://doi.org/10.1038/ncomms6488
Cheeseman, K., & Weitzman, J. B. (2015). Host–parasite interactions: an intimate epigenetic relationship. Cellular Microbiology, 17(8), 1121-1132. https://doi.org/10.1111/cmi.12471 DOI: https://doi.org/10.1111/cmi.12471
Chowdhury, S., Sais, D., Donnelly, S., & Tran, N. (2024). The knowns and unknowns of helminth–host mirna cross-kingdom communication. Trends in Parasitology, 40(2), 176-191. https://doi.org/10.1016/j.pt.2023.12.003 DOI: https://doi.org/10.1016/j.pt.2023.12.003
Coakley, G., Buck, A. H., & Maizels, R. M. (2016). Host parasite communications—Messages from helminths for the immune system: Parasite communication and cell-cell interactions. Molecular and Biochemical Parasitology, 208(1), 33-40. https://doi.org/10.1016/j.molbiopara.2016.06.003 DOI: https://doi.org/10.1016/j.molbiopara.2016.06.003
Del Giudice, M. (2019). Invisible designers: brain evolution through the lens of parasite manipulation. The Quarterly Review of Biology, 94, 249-282. https://doi.org/10.1086/705038 DOI: https://doi.org/10.1086/705038
Duffy, M. A., Brassil, C. E., Hall, S. R., Tessier, A. J., Cáceres, C. E., & Conner, J. K. (2008). Parasite-mediated disruptive selection in a natural Daphnia population. BMC Evolutionary Biology, 8(1), 1-9. https://doi.org/10.1186/1471-2148-8-80 DOI: https://doi.org/10.1186/1471-2148-8-80
Feldmeyer, B., Mazur, J., Beros, S., Lerp, H., Binder, H., & Foitzik, S. (2016). Gene expression patterns underlying parasite‐induced alterations in host behaviour and life history. Molecular Ecology, 25(2), 648-660. https://doi.org/10.1111/mec.13498 DOI: https://doi.org/10.1111/mec.13498
Fitz-James, M. H., & Cavalli, G. (2022). Molecular mechanisms of transgenerational epigenetic inheritance. Nature Reviews Genetics, 23(6), 325-341. https://doi.org/10.1038/s41576-021-00438-5 DOI: https://doi.org/10.1038/s41576-021-00438-5
Floris, I., Kraft, J. D., & Altosaar, I. (2016). Roles of microRNA across prenatal and postnatal periods. International journal of molecular sciences, 17(12), 1994. https://doi.org/10.3390/ijms17121994 DOI: https://doi.org/10.3390/ijms17121994
Frick, A., Björkstrand, J., Lubberink, M., Eriksson, A., Fredrikson, M., & Åhs, F. (2022). Dopamine and fear memory formation in the human amygdala. Molecular Psychiatry, 27(3), 1704-1711. https://doi.org/10.1038/s41380-021-01400-x DOI: https://doi.org/10.1038/s41380-021-01400-x
Gopinath, A., Mackie, P. M., Phan, L. T., Mirabel, R., Smith, A. R., Miller, E., Franks, S., Syed, O., Riaz, T., Law, B. K., Urs, N., & Khoshbouei, H. (2023). Who Knew? Dopamine Transporter Activity Is Critical in Innate and Adaptive Immune Responses. Cells, 12(2), 269. https://doi.org/10.3390/cells12020269 DOI: https://doi.org/10.3390/cells12020269
Gopko, M. V., & Mikheev, V. N. (2019). Parasitic manipulations of the host phenotype: effects in internal and external environments. Biology Bulletin Reviews, 9(1), 1-28. https://doi.org/10.1134/S2079086419010018 DOI: https://doi.org/10.1134/S2079086419010018
Gurtan, A. M., & Sharp, P. A. (2013). The role of miRNAs in regulating gene expression networks. Journal of molecular biology, 425(19), 3582-3600. https://doi.org/10.1016/j.jmb.2013.03.007 DOI: https://doi.org/10.1016/j.jmb.2013.03.007
Hasik, A. Z., Ilvonen, J. J., Gobbin, T. P., Suhonen, J., Beaulieu, J. M., Poulin, R., & Siepielski, A. M. (2025). Parasitism as a driver of host diversification. Nature Reviews Biodiversity 1, 1-10. https://doi.org/10.1038/s44358-025-00045-w DOI: https://doi.org/10.1038/s44358-025-00045-w
He, X., & Pan, W. (2022). Host–parasite interactions mediated by cross-species microRNAs. Trends in parasitology, 38(6), 478-488. https://doi.org/10.1016/j.pt.2022.02.011 DOI: https://doi.org/10.1016/j.pt.2022.02.011
Helluy, S., & Thomas, F. (2003). Effects of Microphallus papillorobustus (Platyhelminthes: Trematoda) on serotonergic immunoreactivity and neuronal architecture in the brain of Gammarus insensibilis (Crustacea: Amphipoda). Proceedings of the Royal Society of London. Series B: Biological Sciences, 270(1515), 563-568. https://doi.org/10.1098/rspb.2002.2264 DOI: https://doi.org/10.1098/rspb.2002.2264
Hernandez-Caballero, I., Garcia-Longoria, L., Gomez-Mestre, I., & Marzal, A. (2022). The adaptive host manipulation hypothesis: parasites modify the behaviour, morphology, and physiology of amphibians. Diversity, 14(9), 739. https://doi.org/10.3390/d14090739 DOI: https://doi.org/10.3390/d14090739
Hughes, D. P., & Libersat, F. (2019). Parasite manipulation of host behavior. Current Biology, 29, R45-R47 (2019). https://doi.org/10.1016/j.cub.2018.12.001 DOI: https://doi.org/10.1016/j.cub.2018.12.001
Jezkova, T., & Wiens, J. J. (2017). What explains patterns of diversification and richness among animal phyla?. The American Naturalist, 189(3), 201-212. https://doi.org/10.1086/690194 DOI: https://doi.org/10.1086/690194
Jovani, R., & Tella, J. L. (2006). Parasite prevalence and sample size: misconceptions and solutions. Trends in parasitology, 22(5), 214-218. https://doi.org/10.1016/j.pt.2006.02.011 DOI: https://doi.org/10.1016/j.pt.2006.02.011
Judice, C. C., Bourgard, C., Kayano, A. C., Albrecht, L., & Costa, F. T. (2016). MicroRNAs in the host-apicomplexan parasites interactions: a review of immunopathological aspects. Frontiers in cellular and infection microbiology, 6, 5. https://doi.org/10.3389/fcimb.2016.00005 DOI: https://doi.org/10.3389/fcimb.2016.00005
Karaer, M. C., Čebulj-Kadunc, N., & Snoj, T. (2023). Stress in wildlife: comparison of the stress response among domestic, captive, and free-ranging animals. Frontiers in Veterinary Science, 10, 1167016. https://doi.org/10.3389/fvets.2023.1167016 DOI: https://doi.org/10.3389/fvets.2023.1167016
Kaushik, M., Lamberton, P. H., & Webster, J. P. (2012). The role of parasites and pathogens in influencing generalised anxiety and predation-related fear in the mammalian central nervous system. Hormones and behavior, 62(3), 191-201. https://doi.org/10.1016/j.yhbeh.2012.04.002 DOI: https://doi.org/10.1016/j.yhbeh.2012.04.002
Love, A. C., & Wagner, G. P. (2022). Co-option of stress mechanisms in the origin of evolutionary novelties. Evolution, 76(3), 394-413. https://doi.org/10.1111/evo.14421 DOI: https://doi.org/10.1111/evo.14421
Matthews, S. G., & Phillips, D. I. (2012). Transgenerational inheritance of stress pathology. Experimental Neurology, 233, 95-101. https://doi.org/10.1016/j.expneurol.2011.01.009 DOI: https://doi.org/10.1016/j.expneurol.2011.01.009
Monsoro-Burq, A. H. (2015). PAX transcription factors in neural crest development. Seminars in Cell & Developmental Biology, 44, 87-96. https://doi.org/10.1016/j.semcdb.2015.09.015 DOI: https://doi.org/10.1016/j.semcdb.2015.09.015
Morgan, H. D., Sutherland, H. G., Martin, D. I., & Whitelaw, E. (1999). Epigenetic inheritance at the agouti locus in the mouse. Nature genetics, 23(3), 314-318. https://doi.org/10.1038/15490 DOI: https://doi.org/10.1038/15490
Nadler, L. E., Adamo, S. A., Hawley, D. M., & Binning, S. A. (2023). Mechanisms and consequences of infection‐induced phenotypes. Functional Ecology, 37(4), 796-800. https://doi.org/10.1111/1365-2435.14309 DOI: https://doi.org/10.1111/1365-2435.14309
Nuismer, S. L., & Otto, S. P. (2005). Host–parasite interactions and the evolution of gene expression. PLoS Biology, 3(7), e203. https://doi.org/10.1371/journal.pbio.0030203 DOI: https://doi.org/10.1371/journal.pbio.0030203
O’Dwyer, K., Dargent, F., Forbes, M. R., & Koprivnikar, J. (2020). Parasite infection leads to widespread glucocorticoid hormone increases in vertebrate hosts: A meta‐analysis. Journal of animal ecology, 89(2), 519-529. https://doi.org/10.1111/1365-2656.13123 DOI: https://doi.org/10.1111/1365-2656.13123
Oleinic, R., Posedi, J., Beck, R., Šprem, N., Škorput, D., Pokorny, B., Škorjanc, D., Prevolnik Povše, M., & Skok, J. (2024). Testing the ‘parasite-mediated domestication’hypothesis: a comparative approach to the wild boar and domestic pig as model species. PeerJ, 12, e18463. https://doi.org/10.7717/peerj.18463 DOI: https://doi.org/10.7717/peerj.18463
Park, Y. (2017). MicroRNA exocytosis by vesicle fusion in neuroendocrine cells. Frontiers in endocrinology, 8, 355. https://doi.org/10.3389/fendo.2017.00355 DOI: https://doi.org/10.3389/fendo.2017.00355
Pasquinelli, A. E. (2012). MicroRNAs and their targets: recognition, regulation and an emerging reciprocal relationship. Nature Reviews Genetics, 13, 271-282. https://doi.org/10.1038/nrg3162 DOI: https://doi.org/10.1038/nrg3162
Paul, S., Ruiz-Manriquez, L. M., Serrano-Cano, F. I., Estrada-Meza, C., Solorio-Diaz, K. A., & Srivastava, A. (2020). Human microRNAs in host–parasite interaction: a review. 3 Biotech, 10, 1-16. https://doi.org/10.1007/s13205-020-02498-6 DOI: https://doi.org/10.1007/s13205-020-02498-6
Poulin, R. (1994). The evolution of parasite manipulation of host behaviour: a theoretical analysis. Parasitology, 109(S1), S109-118. https://doi.org/10.1017/S0031182000085127 DOI: https://doi.org/10.1017/S0031182000085127
Poulin, R., & Thomas, F. (1999). Phenotypic Variability Induced by Parasites: Extent and Evolutionary Implications. Parasitology Today, 15, 28-32. https://doi.org/10.1016/S0169-4758(98)01357-X DOI: https://doi.org/10.1016/S0169-4758(98)01357-X
Poulin, R. (2010). Parasite manipulation of host behavior: an update and frequently asked questions. Advances in the Study of Behavior, 41, 151-186). https://doi.org/10.1016/S0065-3454(10)41005-0 DOI: https://doi.org/10.1016/S0065-3454(10)41005-0
Poulin, R. (2013). Parasite manipulation of host personality and behavioural syndromes. Journal of Experimental Biology, 216(1), 18-26. https://doi.org/10.1242/jeb.073353 DOI: https://doi.org/10.1242/jeb.073353
Rakyan, V. K., Chong, S., Champ, M. E., Cuthbert, P. C., Morgan, H. D., Luu, K. V., & Whitelaw, E. (2003). Transgenerational inheritance of epigenetic states at the murine Axin Fu allele occurs after maternal and paternal transmission. Proceedings of the National Academy of Sciences, 100(5), 2538-2543. https://doi.org/10.1073/pnas.0436776100 DOI: https://doi.org/10.1073/pnas.0436776100
Rojas-Pirela, M., Andrade-Alviárez, D., Quiñones, W., Rojas, M. V., Castillo, C., Liempi, A., Medina, L., Guerrero-Muñoz, J., Fernández-Moya, A., Ortega, Y. A., Araneda, S., Maya, J. D., & Kemmerling, U. (2023). microRNAs: Critical Players during Helminth Infections. Microorganisms, 11(1), 61. https://doi.org/10.3390/microorganisms11010061 DOI: https://doi.org/10.3390/microorganisms11010061
Ruff, J. S., Nelson, A. C., Kubinak, J. L., & Potts, W. K. (2012). MHC signaling during social communication. Advances in Experimental Medicine and Biology, 738, 290-313. https://doi.org/10.1007/978-1-4614-1680-7_17 DOI: https://doi.org/10.1007/978-1-4614-1680-7_17
Sandland, G. J., & Goater, C. P. (2001). Parasite-induced variation in host morphology: brain-encysting trematodes in fathead minnows. Journal of Parasitology, 87(2), 267-272. https://doi.org/10.1645/0022-3395(2001)087[0267:PIVIHM]2.0.CO;2 DOI: https://doi.org/10.1645/0022-3395(2001)087[0267:PIVIHM]2.0.CO;2
Sharma, A. (2014). Novel transcriptome data analysis implicates circulating microRNAs in epigenetic inheritance in mammals. Gene, 538(2), 366-372. https://doi.org/10.1016/j.gene.2014.01.051 DOI: https://doi.org/10.1016/j.gene.2014.01.051
Shaw, T., Barr, F. G., & Üren, A. (2024). The PAX Genes: Roles in Development, Cancer, and Other Diseases. Cancers, 16, 1022. https://doi.org/10.3390/cancers16051022 DOI: https://doi.org/10.3390/cancers16051022
Skinner, M. K. (2014). Environmental stress and epigenetic transgenerational inheritance. BMC Medicine 12, 1-5. https://doi.org/10.1186/s12916-014-0153-y DOI: https://doi.org/10.1186/s12916-014-0153-y
Skok, J. (2023). The parasite-mediated domestication hypothesis. Agricultura Scientia, 20, 1-7. https://doi.org/10.18690/agricsci.20.1.1 DOI: https://doi.org/10.18690/agricsci.20.1.1
Thomas, F., Poulin, R., & Brodeur, J. (2010). Host manipulation by parasites: a multidimensional phenomenon. Oikos, 119(8), 1217-1223. https://doi.org/10.1111/j.1600-0706.2009.18077.x DOI: https://doi.org/10.1111/j.1600-0706.2009.18077.x
Trapezov, O. V. (1987). Селекционное преобразование оборонительной реакции на человека у американской норки. Genetika, 23(6), p.1120.
Trut, L. N. (1999). Early Canid Domestication: The Farm-Fox Experiment: Foxes bred for tamability in a 40-year experiment exhibit remarkable transformations that suggest an interplay between behavioral genetics and development. American Scientist, 87(2), 160-169. DOI: https://doi.org/10.1511/1999.2.160
Trut, L. N., & Kharlamova, A. V. (2020). Domestication as a process generating phenotypic diversity. In Phenotypic Switching (pp. 511-526). Academic Press. DOI: https://doi.org/10.1016/B978-0-12-817996-3.00014-1
van Otterdijk, S. D., & Michels, K. B. (2016). Transgenerational epigenetic inheritance in mammals: how good is the evidence?. The FASEB Journal 30(7), 2457-2465. https://doi.org/10.1096/fj.201500083 DOI: https://doi.org/10.1096/fj.201500083
Wang, S. J., Sharkey, K. A., & McKay, D. M. (2018). Modulation of the immune response by helminths: a role for serotonin?. Bioscience Reports, 38, BSR20180027. https://doi.org/10.1042/BSR20180027 DOI: https://doi.org/10.1042/BSR20180027
Weiner, A. M. (2018). MicroRNAs and the neural crest: from induction to differentiation. Mechanisms of development, 154, 98-106. https://doi.org/10.1016/j.mod.2018.05.009 DOI: https://doi.org/10.1016/j.mod.2018.05.009
Wijayawardena, B. K., Minchella, D. J., & DeWoody, J. A. (2016). The influence of trematode parasite burden on gene expression in a mammalian host. BMC genomics, 17(1), 600. https://doi.org/10.1186/s12864-016-2950-5 DOI: https://doi.org/10.1186/s12864-016-2950-5
Wilkins, A. S., Wrangham, R. W., & Fitch, W. T. (2014). The “domestication syndrome” in mammals: a unified explanation based on neural crest cell behavior and genetics. Genetics, 197, 795-808. https://doi.org/10.1534/genetics.114.165423 DOI: https://doi.org/10.1534/genetics.114.165423
Xu, L., Yang, B. F., & Ai, J. (2013). MicroRNA transport: a new way in cell communication. Journal of cellular physiology, 228(8), 1713-1719. https://doi.org/10.1002/jcp.24344 DOI: https://doi.org/10.1002/jcp.24344
Yao, Q., Chen, Y., & Zhou, X. (2019). The roles of microRNAs in epigenetic regulation. Current Opinion in Chemical Biology, 51, 11-17. https://doi.org/10.1016/j.cbpa.2019.01.024 DOI: https://doi.org/10.1016/j.cbpa.2019.01.024
Zhang, X., Flick, K., Rizzo, M., Pignatelli, M., & Tonegawa, S. (2025). Dopamine induces fear extinction by activating the reward-responding amygdala neurons. Proceedings of the National Academy of Sciences, 122(18), e2501331122. https://doi.org/10.1073/pnas.2501331122 DOI: https://doi.org/10.1073/pnas.2501331122
Zeng, Y., & Wiens, J. J. (2021). Species interactions have predictable impacts on diversification. Ecology Letters 24(2), 239-248. https://doi.org/10.1111/ele.13635 DOI: https://doi.org/10.1111/ele.13635
Zheng, Y., Cai, X., & Bradley, J. E. (2013). microRNAs in parasites and parasite infection. RNA biology, 10(3), 371-379. https://doi.org/10.4161/rna.23716 DOI: https://doi.org/10.4161/rna.23716
Published
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.
License
Copyright (c) 2026 Bio Communications

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Copyright © Bio Communications. This article is published under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) license. Under this license, you are free to share (copy and redistribute) this material in any medium or format for non-commercial purposes, provided you give appropriate credit to the author(s) and the journal. No modifications or adaptations of the material are permitted. The copyright for this article remains with the journal Bio Communications.







 .
.