Unveiling the Hidden Link of Biosecurity in Preventing Vaccine Failure in Livestock: An Updated Review
DOI:
https://doi.org/10.71320/bcs.0003Keywords:
biosecurity measure, vaccination, livestock, challenges, mitigation strategiesAbstract
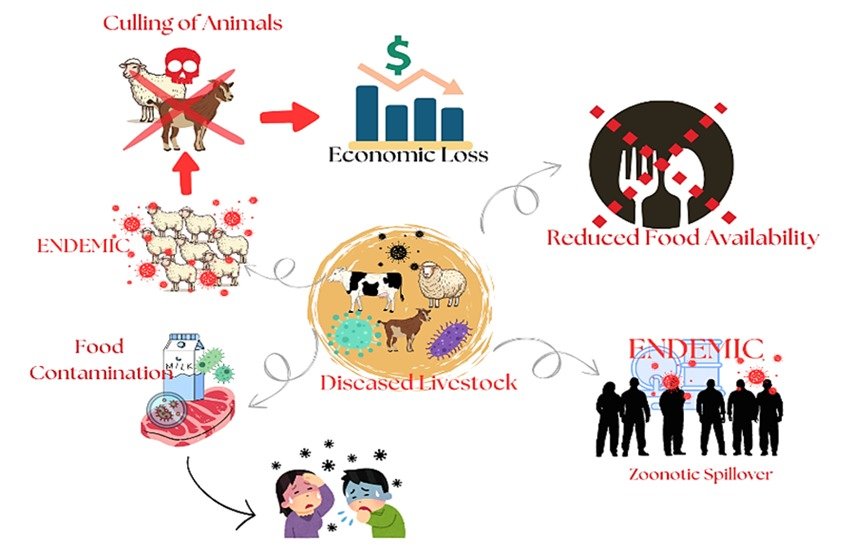
The growing global population has amplified the demand for food, making the international trade of livestock a significant threat to animal and public health due to the increased risk of zoonotic disease transmission through animal movement or the consumption of their products. Biosecurity measures (BSMs) and vaccination are two essential tools that, when effectively implemented, can significantly reduce the spread of diseases from livestock. However, their failure not only escalates the burden of disease but also compromises food security and public health. Weak or poorly enforced BSMs can undermine the efficacy of vaccinations, creating challenges against disease prevention efforts. Conversely, robust BSMs such as nutritional and environmental management, routine monitoring of disease, farm management, and hygiene provide a critical foundation for successful vaccination programs by minimizing disease exposure and ensuring vaccine effectiveness. Inappropriate vaccination administration, inadequate cold chain management, and the frequency of immunosuppressive illnesses are also reported as contributing factors to vaccine failure. This review delves into the interconnected roles of BSMs and vaccination, unveiling the importance of the implementation of biosecurity principles in preventing vaccine failure. It also explores how the integration of biosecurity practices and vaccination strengthens animal health systems, mitigates zoonotic risks, and enhances overall food safety. Future research should concentrate on integrated biosecurity techniques by strengthening on-farm biosecurity protocols, proper handling and administration of vaccines, careful disease surveillance, and awareness programs thereby increasing the resilience of our livestock industry against notable diseases.
References
Admassu, B., Getnet, K., Shite, A., & Mohammed, S. (2015). Review on foot and mouth disease: Distribution and economic significance. Academic Journal of Animal Diseases, 4(3), 160-169. https://doi.org/10.5829/idosi.ajad.2015.4.3.95165
Afzal, H., Umar, M., Hussain, W., & Cheng, L.-T. (2024). Lumpy Skin Disease: An Encroaching Risk In Cattle Farming. Taiwan Veterinary Journal, 49(01), 1-11. https://doi.org/10.1142/S1682648524300016 DOI: https://doi.org/10.1142/S1682648524300016
Ahaduzzaman, M., & Science. (2020). Peste des petits ruminants (PPR) in Africa and Asia: A systematic review and meta‐analysis of the prevalence in sheep and goats between 1969 and 2018. Veterinary medicine international, 6(4), 813-833. https://doi.org/10.1002/vms3.300 DOI: https://doi.org/10.1002/vms3.300
Akar, K., Brangsch, H., Jamil, T., Öz, G. Y., Baklan, E. A., Eroğlu, B., Atıl, E., Gürbilek, S. E., Keskin, O., & Tel, O. Y. (2024). Genomic analysis of Brucella isolates from animals and humans, Türkiye, 2010 to 2020. Eurosurveillance, 29(38), 2400105. https://doi.org/10.2807/1560-7917.ES.2024.29.38.2400105 DOI: https://doi.org/10.2807/1560-7917.ES.2024.29.38.2400105
Al-Kubati, A. A., Hussen, J., Kandeel, M., Al-Mubarak, A. I., & Hemida, M. G. (2021). Recent advances on the bovine viral diarrhea virus molecular pathogenesis, immune response, and vaccines development. Frontiers in veterinary science, 8, 665128. https://doi.org/10.3389/fvets.2021.665128 DOI: https://doi.org/10.3389/fvets.2021.665128
Albina, E., Kwiatek, O., Minet, C., Lancelot, R., de Almeida, R. S., & Libeau, G. (2013). Peste des petits ruminants, the next eradicated animal disease? Veterinary microbiology, 165(1-2), 38-44. https://doi.org/10.1016/j.vetmic.2012.12.013s DOI: https://doi.org/10.1016/j.vetmic.2012.12.013
Ali, I., Rehman, A., Mushtaq, M. H., Ijaz, M., Khaliq, M. S., Khan, M. S. U., Khalid, S., Masud, A., Abbas, A., & Parveen, S. (2022). Outbreak investigation and identification of risk factors associated with the occurrence of foot and mouth disease in Punjab, Pakistan. Preventive Veterinary Medicine, 202, 105613. https://doi.org/10.1016/j.prevetmed.2022.105613 DOI: https://doi.org/10.1016/j.prevetmed.2022.105613
Aslam, M., & Alkheraije, K. A. (2023). The prevalence of foot-and-mouth disease in Asia. Frontiers in veterinary science, 10, 1201578. https://doi.org/10.3389/fvets.2023.1201578 DOI: https://doi.org/10.3389/fvets.2023.1201578
Bonanni, P., Sacco, C., Donato, R., Capei, R., & Infection. (2014). Lifelong vaccination as a key disease-prevention strategy. Clinical Microbiology, 20, 32-36. https://doi.org/10.1111/1469-0691.12537 DOI: https://doi.org/10.1111/1469-0691.12537
Carpenter, T. E., O'Brien, J. M., Hagerman, A. D., & McCarl, B. A. (2011). Epidemic and economic impacts of delayed detection of foot-and-mouth disease: a case study of a simulated outbreak in California. Journal of Veterinary Diagnostic Investigation, 23(1), 26-33. https://doi.org/10.1177/104063871102300104 DOI: https://doi.org/10.1177/104063871102300104
Chakraborty, S., Kumar, A., Tiwari, R., Rahal, A., Malik, Y., Dhama, K., Pal, A., & Prasad, M. (2014). Advances in diagnosis of respiratory diseases of small ruminants. Veterinary medicine international, 2014(1), 508304. https://doi.org/10.1155/2014/508304 DOI: https://doi.org/10.1155/2014/508304
Chauhan, V., & Singh, M. P. (2020). Immuno-informatics approach to design a multi-epitope vaccine to combat cytomegalovirus infection. European Journal of Pharmaceutical Sciences, 147, 105279. https://doi.org/10.1016/j.ejps.2020.105279 DOI: https://doi.org/10.1016/j.ejps.2020.105279
Correia-Gomes, C., Henry, M. K., Auty, H. K., & Gunn, G. J. (2017). Exploring the role of small-scale livestock keepers for national biosecurity—The pig case. Preventive Veterinary Medicine, 145, 7-15. https://doi.org/10.1016/j.prevetmed.2017.06.005 DOI: https://doi.org/10.1016/j.prevetmed.2017.06.005
Crowcroft, N. S., & Klein, N. P. (2018). A framework for research on vaccine effectiveness. Vaccine, 36(48), 7286-7293. https://doi.org/10.1016/j.vaccine.2018.04.016 DOI: https://doi.org/10.1016/j.vaccine.2018.04.016
Das, M., Chowdhury, M. S. R., Akter, S., Mondal, A. K., Uddin, M. J., Rahman, M. M., & Rahman, M. M. (2021). An updated review on lumpy skin disease: perspective of Southeast Asian countries. J adv biotechnol exp ther, 4(3), 322-323. https://doi.org/10.5455/jabet.2021.d13 3 DOI: https://doi.org/10.5455/jabet.2021.d133
Demeli, A., & Fındık, M. (2021). Assessment of cattle and sheep Brucellosis in Turkey. Veteriner Hekimler Derneği Dergisi, 92(1), 7-15. https://doi.org/10.33188/vetheder.678481 DOI: https://doi.org/10.33188/vetheder.678481
Doeschl-Wilson, A., Knap, P., Opriessnig, T., & More, S. J. (2021). Livestock disease resilience: from individual to herd level. Animals, 15, 100286. https://doi.org/10.1016/j.animal.2021.100286 DOI: https://doi.org/10.1016/j.animal.2021.100286
Fayyaz, A., Saleemi, M. K., Gul, S. T., Gilani, M. M., & Irshad, H. (2023). Sero-epidemiology and Pathology of Infectious Bronchitis in Commercial Poultry from Faisalabad Division. Pakistan Veterinary Journal, 43(1). https://doi.org/10.29261/pakvetj/2021.065
Ganter, M. (2015). Zoonotic risks from small ruminants. Veterinary microbiology, 181(1-2), 53-65. https://doi.org/10.1016/j.vetmic.2015.07.015 DOI: https://doi.org/10.1016/j.vetmic.2015.07.015
Gates, M. C., Earl, L., & Enticott, G. (2021). Factors influencing the performance of voluntary farmer disease reporting in passive surveillance systems: a scoping review. Preventive Veterinary Medicine, 196, 105487. https://doi.org/10.1016/j.prevetmed.2021.105487 DOI: https://doi.org/10.1016/j.prevetmed.2021.105487
Gupta, T., Patial, V., Bali, D., Angaria, S., Sharma, M., & Chahota, R. J. V. r. c. (2020). A review: Lumpy skin disease and its emergence in India. 44, 111-118. https://doi.org/10.1007/s11259-020-09780-1 DOI: https://doi.org/10.1007/s11259-020-09780-1
Heininger, U., Bachtiar, N., Bahri, P., Dana, A., Dodoo, A., Gidudu, J., & Dos Santos, E. M. (2012). The concept of vaccination failure. Vaccines, 30(7), 1265-1268. https://doi.org/10.1016/j.vaccine.2011.12.048
Heininger, U., Bachtiar, N. S., Bahri, P., Dana, A., Dodoo, A., Gidudu, J., & Dos Santos, E. M. (2012). The concept of vaccination failure. Vaccine, 30(7), 1265-1268. https://doi.org/10.1016/j.vaccine.2011.12.048 DOI: https://doi.org/10.1016/j.vaccine.2011.12.048
Hennessy, D. A., & Marsh, T. L. (2021). Economics of animal health and livestock disease. Handbook of agricultural economics, 5, 4233-4330. https://doi.org/10.1016/bs.hesagr.2021.10.005 DOI: https://doi.org/10.1016/bs.hesagr.2021.10.005
Hill, E. M., Prosser, N. S., Ferguson, E., Kaler, J., Green, M. J., Keeling, M. J., & Tildesley, M. J. (2022). Modelling livestock infectious disease control policy under differing social perspectives on vaccination behaviour. PLOS Computational Biology, 18(7), e1010235. https://doi.org/10.1371/journal.pcbi.1010235 DOI: https://doi.org/10.1371/journal.pcbi.1010235
Huber, N., Andraud, M., Sassu, E. L., Prigge, C., Zoche-Golob, V., Käsbohrer, A., d'Angelantonio, D., Viltrop, A., Żmudzki, J., & Jones, H. (2022). What is a biosecurity measure? A definition proposal for animal production and linked processing operations. One Health, 15, 100433. https://doi.org/10.1016/j.onehlt.2022.100433 DOI: https://doi.org/10.1016/j.onehlt.2022.100433
Hussain, M. M., Khalid, A., Rehman, M. U., & Wahab, A. (2024). Lumpy skin disease: an emerging concern in Pakistan and its impact on national economic loss. BioScientific Review, 6(2), 121-135. https://doi.org/10.32350/bsr.62.iv DOI: https://doi.org/10.32350/bsr.62.iv
Ismail, S., Ahmad, S., & Azam, S. S. (2020). Immunoinformatics characterization of SARS-CoV-2 spike glycoprotein for prioritization of epitope based multivalent peptide vaccine. Journal of Molecular Liquids, 314, 113612. https://doi.org/10.1016/j.molliq.2020.113612 DOI: https://doi.org/10.1016/j.molliq.2020.113612
Khan, K., Yaqub, T., Shabbir, M. Z., Aslam, A., Mukhtar, N., Fazal, S., Iftikhar, R., & Hassan, M. (2023). In-silico vaccine matching and its validation through in-vivo immune protection analysis for imported and indigenous vaccines against recent field isolate of avian influenza H9N2. Veterinary Vaccine, 2(2), 100029. https://doi.org/10.1016/j.vetvac.2023.100029 DOI: https://doi.org/10.1016/j.vetvac.2023.100029
Khurana, S. K., Sehrawat, A., Tiwari, R., Prasad, M., Gulati, B., Shabbir, M. Z., Chhabra, R., Karthik, K., Patel, S. K., & Pathak, M. (2021). Bovine brucellosis–a comprehensive review. Veterinary Quarterly, 41(1), 61-88. DOI: https://doi.org/10.1080/01652176.2020.1868616
Kumar, R., Singh, S., & Savalia, C. (2015). Overview of emerging zoonoses in India: Areas of concern. Journal of Troicalp Diseases, 3(165), 2. https://doi.org/10.4172/2329-891X.1000165 DOI: https://doi.org/10.4172/2329-891X.1000165
Layton, D. S., Choudhary, A., & Bean, A. G. (2017a). Breaking the chain of zoonoses through biosecurity in livestock. Vaccines, 35(44), 5967-5973. https://doi.org/10.1016/j.vaccine.2017.07.110
Layton, D. S., Choudhary, A., & Bean, A. G. (2017b). Breaking the chain of zoonoses through biosecurity in livestock. Vaccinez, 35(44), 5967-5973. https://doi.org/10.1016/j.vaccine.2017.07.110 DOI: https://doi.org/10.1016/j.vaccine.2017.07.110
Léger, A., De Nardi, M., Simons, R., Adkin, A., Ru, G., Estrada-Peña, A., & Stärk, K. D. (2017). Assessment of biosecurity and control measures to prevent incursion and to limit spread of emerging transboundary animal diseases in Europe: An expert survey. Vaccines, 35(44), 5956-5966. https://doi.org/10.1016/j.vaccine.2017.07.034 DOI: https://doi.org/10.1016/j.vaccine.2017.07.034
Leslie, J. (2000). Newcastle disease: outbreak losses and control policy costs. Veterinary Record, 146(21), 603-606. https://doi.org/10.1136/vr.146.21.603 DOI: https://doi.org/10.1136/vr.146.21.603
Manuja, B. K., Manuja, A., & Singh, R. K. (2014). Globalization and livestock biosecurity. Agricultural Research, 3, 22-31. https://doi.org/10.1007/s40003-014-0097-7 DOI: https://doi.org/10.1007/s40003-014-0097-7
Masood, I., Tahir, M. J., Naeem, A., Shrateh, O. N., & Ahmed, A. (2023). The new wave of Congo virus in Pakistan: emerging threat. Tropical Medicine Health, 51(1), 62. https://doi.org/10.1186/s41182-023-00559-z DOI: https://doi.org/10.1186/s41182-023-00559-z
Mishra, J., Mishra, P., & Arora, N. K. (2021). Linkages between environmental issues and zoonotic diseases: with reference to COVID-19 pandemic. Environmental Sustainability, 4(3), 455-467. https://doi.org/10.1007/s42398-021-00165-x DOI: https://doi.org/10.1007/s42398-021-00165-x
Morris, G., Ehlers, S., & Shutske, J. (2023). US small-scale livestock operation approach to biosecurity. Agriculture, 13(11), 2086. https://doi.org/10.3390/agriculture13112086 DOI: https://doi.org/10.3390/agriculture13112086
Msimang, V., Rostal, M. K., Cordel, C., Machalaba, C., Tempia, S., Bagge, W., Burt, F. J., Karesh, W. B., Paweska, J. T., & Thompson, P. N. (2022). Factors affecting the use of biosecurity measures for the protection of ruminant livestock and farm workers against infectious diseases in central South Africa. Transboundary Emerging Diseases, 69(5), e1899-e1912. https://doi.org/10.1111/tbed.14525 DOI: https://doi.org/10.1111/tbed.14525
Nandi, A., & Allen, L. J. (2021). Probability of a zoonotic spillover with seasonal variation. Infectious Disease Modelling, 6, 514-531. https://doi.org/10.1016/j.idm.2021.01.013 DOI: https://doi.org/10.1016/j.idm.2021.01.013
Niemi, J. K., Sahlström, L., Kyyrö, J., Lyytikäinen, T., & Sinisalo, A. (2016). Farm characteristics and perceptions regarding costs contribute to the adoption of biosecurity in Finnish pig and cattle farms. Review of Agricultural, Food Environmental Studies, 97, 215-223. https://doi.org/10.1007/s41130-016-0022-5 DOI: https://doi.org/10.1007/s41130-016-0022-5
Nuvey, F. S., Fink, G., Hattendorf, J., Mensah, G. I., Addo, K. K., Bonfoh, B., & Zinsstag, J. (2023). Access to vaccination services for priority ruminant livestock diseases in Ghana: Barriers and determinants of service utilization by farmers. Preventive Veterinary Medicine, 215, 105919. https://doi.org/10.1016/j.prevetmed.2023.105919 DOI: https://doi.org/10.1016/j.prevetmed.2023.105919
Oluwadare, F. A., Mkpuma, N., Killo, A. O., Inuwa, B., Fagbohun, O., & Meseko, C. A. (2024). Vaccine Quality Challenges in Secretly Traded H5 and H9 Avian Influenza Vaccines in Nigeria. Nigerian Veterinary Journal, 45(3), 33-42. https://doi.org/10.4314/nvj.v45i3.3 DOI: https://doi.org/10.4314/nvj.v45i3.3
Park, J.-H., Lee, K.-N., Ko, Y.-J., Kim, S.-M., Lee, H.-S., Shin, Y.-K., Sohn, H.-J., Park, J.-Y., Yeh, J.-Y., & Lee, Y.-H. (2013). Control of foot-and-mouth disease during 2010–2011 epidemic, South Korea. Emerging infectious diseases, 19(4), 655. https://doi.org/10.3201/eid1904.121320 DOI: https://doi.org/10.3201/eid1904.121320
Perry, B., & Grace, D. (2009). The impacts of livestock diseases and their control on growth and development processes that are pro-poor. Philosophical Transactions of the Royal Society B: Biological Sciences, 364(1530), 2643-2655. https://doi.org/10.1098/rstb.2009.0097 DOI: https://doi.org/10.1098/rstb.2009.0097
Powell, N. D., Allen, R. G., Hufnagle, A. R., Sheridan, J. F., & Bailey, M. T. (2011). Stressor-induced alterations of adaptive immunity to vaccination and viral pathogens. Immunology Allergy Clinics, 31(1), 69-79. https://doi.org/10.1016/j.iac.2010.09.002 DOI: https://doi.org/10.1016/j.iac.2010.09.002
Pulendran, B., & Ahmed, R. (2011). Immunological mechanisms of vaccination. Nature Immunology, 12(6), 509-517. https://doi.org/10.1038/ni.2039 DOI: https://doi.org/10.1038/ni.2039
Raja Sekhara Rao, P., & Naresh Kumar, M. (2015). A dynamic model for infectious diseases: The role of vaccination and treatment. Chaos, Solitons & Fractals, 75, 34-49. https://doi.org/10.1016/j.chaos.2015.02.004 DOI: https://doi.org/10.1016/j.chaos.2015.02.004
Renault, V., Humblet, M.-F., Pham, P. N., & Saegerman, C. (2021). Biosecurity at cattle farms: Strengths, weaknesses, opportunities and threats. Pathogens, 10(10), 1315. https://doi.org/10.3390/pathogens10101315 DOI: https://doi.org/10.3390/pathogens10101315
Robertson, I. D. (2020). Disease Control, Prevention and On-Farm Biosecurity: The Role of Veterinary Epidemiology. Engineering, 6(1), 20-25. https://doi.org/10.1016/j.eng.2019.10.004 DOI: https://doi.org/10.1016/j.eng.2019.10.004
Rodolakis, A. (2014). Zoonoses in goats: How to control them. Small Ruminant Research, 121(1), 12-20. https://doi.org/10.1016/j.smallrumres.2014.01.007 DOI: https://doi.org/10.1016/j.smallrumres.2014.01.007
Rosini, R., Nicchi, S., Pizza, M., & Rappuoli, R. (2020). Vaccines Against Antimicrobial Resistance [Review]. Frontiers in immunology, 11. https://doi.org/10.3389/fimmu.2020.01048 DOI: https://doi.org/10.3389/fimmu.2020.01048
Roth, J. A. (2011). Veterinary Vaccines and Their Importance to Animal Health and Public Health. Procedia in Vaccinology, 5, 127-136. https://doi.org/10.1016/j.provac.2011.10.009 DOI: https://doi.org/10.1016/j.provac.2011.10.009
Saminathan, M., Rana, R., Ramakrishnan, M. A., Karthik, K., Malik, Y. S., Dhama, K. J. J. o. E. B., & Sciences, A. (2016). Prevalence, diagnosis, management and control of important diseases of ruminants with special reference to Indian scenario. Journal of Experimental Biology Agricultural Sciences, 4(3S), 338-367. https://dx.doi.org/10.18006/2016.4(3S).338.367 DOI: https://doi.org/10.18006/2016.4(3S).338.367
Shahzad, R., Irshad, S., Javaid, A., & Yaqoob, T. (2020). Mutational Analysis of Neuraminidase of Avian Influenza virus H9N2 Indicating the Cause of Hyper Pathogenicity in Poultry. Pakistan Veterinary Journal, 40(2). https://doi.org/10.29261/pakvetj/2020.017 DOI: https://doi.org/10.29261/pakvetj/2020.017
Shortall, O., Green, M., Brennan, M., Wapenaar, W., & Kaler, J. (2017). Exploring expert opinion on the practicality and effectiveness of biosecurity measures on dairy farms in the United Kingdom using choice modeling. Journal of dairy science, 100(3), 2225-2239. https://doi.org/10.3168/jds.2016-11435 DOI: https://doi.org/10.3168/jds.2016-11435
Simon-Grifé, M., Martín-Valls, G., Vilar, M., García-Bocanegra, I., Martín, M., Mateu, E., & Casal, J. (2013). Biosecurity practices in Spanish pig herds: perceptions of farmers and veterinarians of the most important biosecurity measures. Preventive veterinary medicine, 110(2), 223-231. https://doi.org/10.1016/j.prevetmed.2012.11.028 DOI: https://doi.org/10.1016/j.prevetmed.2012.11.028
Singh, R. K., Sharma, G. K., Mahajan, S., Dhama, K., Basagoudanavar, S. H., Hosamani, M., Sreenivasa, B., Chaicumpa, W., Gupta, V. K., & Sanyal, A. (2019). Foot-and-mouth disease virus: immunobiology, advances in vaccines and vaccination strategies addressing vaccine failures—an Indian perspective. Vaccines, 7(3), 90. https://doi.org/10.3390/vaccines7030090 DOI: https://doi.org/10.3390/vaccines7030090
Stokstad, M., Klem, T. B., Myrmel, M., Oma, V. S., Toftaker, I., Østerås, O., & Nødtvedt, A. (2020). Using biosecurity measures to combat respiratory disease in cattle: The Norwegian control program for bovine respiratory syncytial virus and bovine coronavirus. Frontiers in Veterinary Science, 7, 167. https://doi.org/10.3389/fvets.2020.00167 DOI: https://doi.org/10.3389/fvets.2020.00167
Tomley, F. M., & Shirley, M. W. (2009). Livestock infectious diseases and zoonoses. Philosophical Transactions of the Royal Society B: Biological Sciences, 364(1530), 2637-2642. https://doi.org/10.1098/rstb.2009.0133 DOI: https://doi.org/10.1098/rstb.2009.0133
Wright, B. K., Jorgensen, B. S., & Smith, L. D. (2018). Understanding the biosecurity monitoring and reporting intentions of livestock producers: identifying opportunities for behaviour change. Preventive Veterinary Medicine, 157, 142-151. https://doi.org/10.1016/j.prevetmed.2018.07.007 DOI: https://doi.org/10.1016/j.prevetmed.2018.07.007
Yohannes, S., Michael, B. G., & Yadeta, W. (2020). Veterinary Vaccines Handling, Transportation and Storage: Factors Challenging their Efficacy and Their Adverse Effects to the Host. Global Veterinaria. http://doi.org/10.5829/idosi.gv.2020.121.127
Yumuk, Z., & O’Callaghan, D. J. I. J. o. I. D. (2012). Brucellosis in Turkey—an overview. International Journal of Infectious Diseases, 16(4), e228-e235. https://doi.org/10.1016/j.ijid.2011.12.011 DOI: https://doi.org/10.1016/j.ijid.2011.12.011
Zafar, S., Sarfraz, M. S., Ali, S., Saeed, L., Mahmood, M. S., Khan, A. U., & Anwar, M. N. J. V. S. (2024). Recapitulation of Peste des Petits Ruminants (PPR) prevalence in small ruminant populations of Pakistan from 2004 to 2023: a systematic review and meta-analysis. 11(6), 280. https://doi.org/10.3390/vetsci11060280 DOI: https://doi.org/10.3390/vetsci11060280
Zanon, T., Alrhmoun, M., & Gauly, M. (2024). Assessing the impact of biosecurity practices and animal welfare in small-scale mountain dairy farming. Scientific Reports, 14(1), 13294. http://doi.org/10.1038/s41598.024.63841.y DOI: https://doi.org/10.1038/s41598-024-63841-y
Zhang, Y.-h., Li, C.-S., Liu, C.-C., & Chen, K. Z. J. R. i. v. s. (2013). Prevention of losses for hog farmers in China: Insurance, on-farm biosecurity practices, and vaccination. 95(2), 819-824. https://doi.org/10.1016/j.rvsc.2013.06.002 DOI: https://doi.org/10.1016/j.rvsc.2013.06.002
Zimmermann, P., & Curtis, N. (2019). Factors that influence the immune response to vaccination. Clinical microbiology reviews, 32(2), 10.1128/cmr. 00084-00018. https://doi.org/10.1128/cmr.00084-18 DOI: https://doi.org/10.1128/CMR.00084-18
Published
License
Copyright (c) 2025 Bio Communications

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Copyright © Bio Communications. This article is published under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) license. Under this license, you are free to share (copy and redistribute) this material in any medium or format for non-commercial purposes, provided you give appropriate credit to the author(s) and the journal. No modifications or adaptations of the material are permitted. The copyright for this article remains with the journal Bio Communications.







 .
.